Do Adenovirus Vaccines Cause Clotting?
The FLARE Four
- The Johnson & Johnson COVID-19 vaccine in the U.S. was paused for two weeks while the CDC and FDA investigated cases of rare and abnormal blood clotting termed "Vaccine-Induced Thrombotic Thrombocytopenia" (VITT). AstraZeneca was similarly investigated in Europe
- Most of the patients who developed this rare clotting condition following either Johnson & Johnson or AstraZeneca vaccination have been women under 50 years of age. These cases have similarities to a syndrome known as heparin-induced thrombocytopenia
- Preliminary studies suggest that certain components of the vaccine may be triggering the immune response that leads to this syndrome, however further studies are needed to determine whether or how the vaccines are causing the clotting disorder
- While the risk of developing VITT after vaccination does appear to be higher in younger women, the risk of VITT is still much lower than the risks associated with COVID-19 infection. These vaccines will still be a critical part of controlling the pandemic
Subscribe to the latest updates from FLARE Advances in Motion
Many people are asking...do adenovirus vaccines cause clotting?
On April 13, 2021, the U.S. Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) recommended pausing the use of the Johnson & Johnson (J&J) vaccine in order to investigate reports of a rare but serious clotting disorder. On April 23, 2021, an advisory panel to the CDC voted to recommend lifting the pause but adding a warning about the clotting disorder, which they have termed "thrombosis with thrombocytopenia syndrome" (TTS). In the U.S., as of May 12, 2021, there are a total of 28 confirmed cases of TTS; 22 were women (18 were between 18 and 49 years old), and all were within 15 days of receiving the vaccine.
European regulators have been investigating similar reports of rare and abnormal clotting in people who received the AstraZeneca vaccine (AstraZeneca is not yet authorized for use in the U.S.), which, like the J&J vaccine, uses an adenovirus-based platform. The vaccine-related clotting events are of an unusual type, with a predominance of cerebral venous thrombosis.
Vaccine Strategy and Production
Both the J&J and AstraZeneca vaccines use an adenovirus-based platform to deliver the genetic information for the spike glycoprotein of SARS-CoV-2, though the two vaccines use different viral vectors with distinct cellular entry receptors. Wild-type adenoviruses are one of the viruses that can cause the common cold and are distinct from SARS-CoV-2, the coronavirus that causes COVID-19. AstraZeneca uses a replication-incompetent chimpanzee adenovirus vector, while J&J uses a replication-incompetent human adenovirus type 26 vector; this is the same vector J&J uses in their Ebola vaccine, which was approved in 2020. While there are many preclinical and clinical trials investigating adenovirus-based vaccines, J&J's Ebola vaccine is the only one currently fully approved, and four adenovirus-based vaccines for COVID-19 have received emergency authorization (Russia's Sputnik V and China's CanSino vaccines are the other two adenovirus-based COVID-19 vaccines currently being administered, albeit not in the U.S.).
Adenoviruses have long been used in molecular biology research, as they can carry a relatively large DNA payload, do not integrate DNA into host genomes and can infect both dividing and non-dividing cells. Adenoviruses have also been investigated for gene therapy. To produce adenoviruses for clinical use, large amounts of plasmid DNA (i.e., circular DNA strands used by bacteria) containing certain viral information and the desired gene of interest (e.g., spike glycoprotein of SARS-CoV-2) is produced and isolated from bacteria. These plasmids have been engineered to remove the E1 region of the adenovirus genome, which contains the genes required for viral replication; this means that the viral particles produced from these plasmids are unable to replicate. Next, the plasmids are transfected (inserted) into human cell lines, which have been engineered to include the missing genes necessary for virus replication and production of viral particles. AstraZeneca uses HEK293 cells (derived from human embryonic kidney cells), and J&J uses PER.C6 cells (derived from human embryonic retinal cells) for this step. Once the viral particles are produced, the viral particles are isolated through a series of filtration and purification steps before being packaged for use.
AstraZeneca
As of April 4, 2021, European regulators had received reports of 222 cases of blood clotting problems (169 cases of cerebral venous sinus thrombosis, CVST, 53 cases of splanchnic vein thrombosis), out of approximately 34 million people who had received the AstraZeneca vaccine, giving an incidence rate of approximately 1 in 100,000 recipients. European regulators say that as of March 22, 2021, they had carried out detailed reviews of 86 cases (62 cases of CVST, 24 cases of splanchnic vein thrombosis), 18 of which had been fatal. On April 7, 2021, the European Medicines Agency's safety committee concluded that unusual blood clots with low blood platelets should be listed as a very rare side effect of the AstraZeneca vaccine. Many countries have recommended that AstraZeneca only be administered to older age groups, and some have stopped administering it entirely. In the U.K., the Joint Committee on Vaccination and Immunisation has recommended that people under 30 years of age be offered an alternative COVID-19 vaccine, as they appeared to be at higher risk for developing thrombosis/thrombocytopenia and relatively lower risk for developing severe COVID-19. It is not clear whether women are at greater risk of developing blood clots after vaccination, or whether they are simply overrepresented among AstraZeneca vaccine recipients, which in many countries tended to be health care workers, who are often young women.
Two case series published in the New England Journal of Medicine (NEJM) on April 9, 2021, report detailed observations of patients in Norway (Schultz et al 2021), Germany and Austria (Greinacher et al 2021), who developed thrombotic thrombocytopenia after receiving the AstraZeneca vaccine. In both case series, patients presented with thrombosis (predominantly CVST, but also in other parts of the body) and thrombocytopenia. These patients also showed high levels of IgG antibodies to platelet factor 4 (PF4)-polyanion complexes and functional activity assays of patient serum showed that platelets in many of the patients were activated and aggregated at baseline.
The authors of both papers speculate that the observed clotting condition is an immune response, similar to a rare reaction to the drug heparin, called heparin-induced thrombocytopenia. Heparin-induced thrombocytopenia is a syndrome characterized by a decrease in platelet count after beginning heparin, and has been reported in up to 5% of patients treated with heparin. PF4, a chemokine released from activated platelets during platelet aggregation, can bind to polyanions, such as heparin or lipopolysaccharides on bacteria, and undergo a conformational change. B lymphocytes generate antibodies against this PF4-anion complex, which is beneficial as a bacterial-host defense mechanism when PF4 is bound to the surface of bacteria, but if this occurs with heparin treatment, it can lead to strong platelet activation and aggregation, or the formation of blood clots.
In both cohorts, patients had high levels of IgG antibodies to PF4-polyanion complexes but had no prior history of heparin treatment. Both Schultz and Greinacher and colleagues termed this condition, "vaccine-induced immune thrombotic thrombocytopenia" (VITT). Both report that treatment with intravenous immune globulin, which inhibits the Fcγ receptor–mediated platelet activation, successfully improved platelet counts and recommend treatment with non-heparin anticoagulants.
Johnson & Johnson
The FDA briefing document of the Phase 3 trial data that led to the issuance of the emergency use authorization noted a slight numerical imbalance of embolic and thrombotic events in the vaccine group (15 events in 14 participants; 0.06% of the vaccine group) as compared to the placebo group (10 events in 10 participants; 0.05% of the placebo group). While only 2 of the events (1 vaccine, 1 placebo, both developing deep vein thrombosis, DVT) were considered by study investigators to be related to the study, the FDA recommended surveillance for further evaluation of thromboembolic events with the deployment of the vaccine in larger populations, to determine if there is a causal relationship between the vaccine and thromboembolic events. During the Advisory Committee on Immunization Practices (ACIP) meeting on April 14, 2021, two cases of CVST were noted from the Phase 3 J&J trial: a 25-year-old man in the vaccine group, and a 24-year-old woman in the placebo group. The man developed symptoms after injection, showed low platelet counts (64,000) and tested positive for antibodies against PF4. The woman in the placebo group developed symptoms more than 50 days after injection, had normal platelet counts and no antibodies against PF4.
J&J vaccines began to be administered in the U.S. on March 2, 2021, and the first case of cerebral thrombosis was reported on March 19. As of May 12, 2021, 28 cases of thrombosis with thrombocytopenia syndrome were identified, 22 in women, all within 3-15 days of vaccination. All of the patients received the vaccine before the April 13 pause. A case series describing 12 of those patients was recently published in JAMA (See et al., 2021). Similar to the case series reports for patients receiving the AstraZeneca vaccine, these patients presented with thrombosis, predominantly CVST, thrombocytopenia and antibodies against PF4. As of May 12, 2021, three patients died, four remain hospitalized including one in an intensive care unit.
AstraZeneca |
Johnson & Johnson |
||
|---|---|---|---|
| |
Schultz et al |
Greinacher et al |
CDC ACIP May 12 Presentation |
| Location |
Oslo University Hospital, Norway |
Germany, Austria |
United States |
Patient Demographics |
|||
| # patients characterized |
5 | 11 + Bloodwork from additional 17 patients; no further clinical data available |
28 |
| Women |
4 |
9 |
22 |
| Age range, years |
32-54 |
22-49 |
18-59 |
| Active hormonal therapy or contraception |
3 |
3 |
4 |
| Days from vaccination to symptom onset |
7-10 |
5-16 |
3-15 |
Clinical Data |
|||
Platelet count nadir, range, /mm3 |
10,000 – 70,000 |
9,000 – 107,000 |
< 50,000 : n=18 50-< 100,000: n=6 100,000-149,000 n=4 |
| # patients with antibodies against PF4 |
5 |
9 (Data not available for 2 patients) |
24 (Data not available for 2 patients; 2 patients tested negative) |
| Cerebral venous sinus thrombosis |
4 |
9 |
19 |
| Patient outcomes |
3 deaths |
6 deaths |
3 deaths |
What Is Triggering This Clotting Reaction?
At this point it is not clear what is triggering VITT. Greinacher and colleagues, who had published the case series of AstraZeneca recipients in Germany and Austria, recently released a preprint where they speculate that non-viral components in the AstraZeneca vaccine could contribute to platelet activation. The researchers first showed that the addition of PF4 to the vaccine viral particles formed aggregates, which increased in size in the presence of anti-PF4 IgG, and could be dissociated with 100IU/mL heparin. 1H-NMR spectrometry identified known excipients such as EDTA (Disodium edetate dihydrate), as well as 70-80ug of protein/mL vaccine in four lots tested. Proteomic analysis revealed 43%-60% of the proteins were human proteins, originating from the HEK293 cells used for virus production; the rest were predominantly adenovirus vector proteins. They showed that 2uM EDTA induced platelet activation in whole blood of healthy volunteers, and both EDTA alone and the AstraZeneca vaccine induced skin edema formation and vascular leakage in wild-type mice. When purified neutrophils were incubated with PF4 and serum from VITT patients, prothrombotic neutrophil extracellular traps (NETs; NETosis) were formed, and these were enhanced in the presence of platelets. They also found that extracellular DNases, which normally degrade NETs, were lower in the serum of VITT patients, compared to healthy controls.
Based on this data, Greinacher and colleagues speculate that upon intramuscular injection, the vaccine and its components activate platelets, releasing PF4, which then binds to vaccine components and viral proteins, forming large aggregates. Interaction between PF4, activated platelets and IgG antibodies leads to NETosis. Meanwhile, EDTA increases capillary leakage at the inoculation site, allowing proteins from the vaccine to enter the blood and activate an inflammatory response. Together, this results in a marked activation of the coagulation system.
Greinacher and colleagues were unable to perform comparative tests with the J&J vaccine, as it was not yet in use in Germany at the time of the study, although they have requested samples for analysis. It's not clear what the implications are for the presence of 35-40ug human cell culture proteins per vaccination dose or how this compares with J&J or other vaccines; other vaccines also contain human proteins, usually listed as amounts of 5ug/mL or less, although it is not always specified. J&J vaccine is not reported to contain EDTA. Adenoviruses are known to potentially stimulate a strong innate and adaptive immune response, and in the context of gene therapy development, much work has been done to modify adenovirus vectors to reduce the immune response (Gregory et al, 2011.) It has been shown however that adenovirus particles can induce the innate immune response even after irradiation to inhibit gene expression. Thus the adenovirus vaccine itself could prompt an exuberant immune response and may interact with other factors in a very small number of individuals to trigger VITT.
Sputnik V, developed by the Russian Gamaleya National Research Institute of Epidemiology and Microbiology, and CanSino, developed by the Chinese company CanSino Biologics, are two other COVID-19 vaccines that use an adenovirus platform. The European Medicines Agency (EMA) is in the early stages of reviewing the safety data for Sputnik V, and so far neither company has reported cases of clotting in vaccine recipients. The EMA has requested AstraZeneca to conduct investigations into identifying the effect of the vaccine on blood clotting and identifying potential risk factors. Additionally, the EMA is supporting projects by two academic consortia centered in the Netherlands aiming to determine if there is a real link between the vaccine and VITT, and identify at-risk populations. A consortia led by Eric C. M. van Gorp at Erasmus University Medical Center in Rotterdam has already been working on studying the effects of coronavirus on blood coagulation.
What About mRNA Vaccines or Other Causes of Blood Clots?
As of May 7, 2021, there have been no reports to VAERS (Vaccine Adverse Event Reporting System) of TTS in people receiving the Pfizer vaccine (135.7 million doses administered), nor in people receiving the Moderna vaccine (110.1 million doses administered). There have been three reports of CVST in people receiving the Moderna vaccine, but all three cases reported normal platelet counts (150,000-450,000/mm3). The Vaccine Safety Datalink, a collaborative project between the CDC's Immunization Safety Office and nine health care organization, has identified 10 total cases of CVST following mRNA vaccine administration out of 5.2 million doses; based on medical history, five cases were ruled unrelated to the vaccine, and five cases were potentially related, but none of those potential cases of CVST coincided with thrombocytopenia.
Estimates of the prevalence of CVST vary, ranging from approximately 0.2-1.57 cases per 100,000 person-years (Stam 2005; Devasagayam et al., 2016), with some studies estimating higher prevalence in women compared to men (Stam 2005), and others putting the risk approximately equal (Devasagayam et al. 2016). In a presentation to the ACIP on April 23, 2021, Johnson & Johnson officials estimated the background rate of CVST with thrombocytopenia to be about 0.1 per million people. A recent preprint analyzed electronic medical records of 601,741 patients who were vaccinated in the Mayo Clinic health system between January 1, 2017, and April 15, 2021, and also received at least one SARS-CoV-2 PCR test (Pawlowski et al 2021). They identified 165 cases of CVST since January 2017, with four patients who also had thrombocytopenia, none of whom were within 30 days of any vaccination, giving a background incidence rate of CVST with thrombocytopenia of two per million patient-years.
With 28 cases of thrombosis with thrombocytopenia out of a total of 8.73 million people vaccinated with J&J, that gives an overall rate of 3.2 cases per million; still very rare, but potentially much higher than the background rate. Breaking the cases down by gender and age however shows that in women aged 30-49 years old, the reporting rate has been 9.4-12.4 cases per million people. Meanwhile, six cases have been reported in men, ranging in age from 18-64 years old, giving a rate of 1.3-2.8 cases per million people. The infection fatality ratio (IFR; the ratio of COVID-19-associated deaths to the total number of SARS-CoV-2 infections) has been estimated to be < 0.1% of people under 50 (O'Driscoll et al, 2020; Perez-Saez et al. 2021), which raises the question of the risk-benefit ratio of the adenovirus vaccines in younger people.
There have been multiple case reports of CVST and other thromboembolic events in patients with COVID-19, although estimates of the prevalence vary widely. One retrospective observational registry study of patients evaluated and/or admitted to an emergency department with COVID-19 reported three CVST patients out of 14,483, giving an incidence of 20 per 100,000 (Siegler et al 2020). A recent preprint from Taquet and colleagues at the University of Oxford examined TriNetX electronic medical records from 513,284 patients, primarily in the U.S., with a confirmed diagnosis of COVID-19, and calculated an absolute risk of 39 CVST cases per million people within two weeks following COVID-19 diagnosis, compared to an overall incidence in the TriNetX records of 0.41 per million people over any two-week period. Worth noting that the authors report that in their TriNetX dataset, two people developed CVST after receiving an mRNA vaccine; one received the Pfizer vaccine, and the other is unknown whether they received Pfizer or Moderna, however as noted above, there are no reports in VAERS of CVST after Pfizer vaccination.
There have been many comparisons about the incidence of blood clots following the AstraZeneca or J&J vaccines, versus the incidence of blood clots in women taking hormonal contraceptives. The FDA has estimated that 1-5 in 10,000 women who are not pregnant and not taking combination oral contraceptives may develop blood clots, and that increases to 3-9 in 10,000 for combination oral contraceptive users, 5-20 out of 10,000 for women who are pregnant and up to 40-65 in 10,000 12 weeks postpartum—much higher than the estimated incidence following vaccination. However, a major difference is the type of blood clots that are common in these conditions. The most common types of clot associated with birth control are deep vein thrombosis (i.e., leg vein) and pulmonary embolism, which are typically treated with blood thinners like heparin. The majority of clots observed following vaccination have been cerebral, with a potentially worse prognosis. Moreover, unlike DVT in association with oral contraception, vaccine-related clots are associated with PF4 antibodies and platelet activation. Health officials recommend non-heparin anticoagulants as heparin may exacerbate the condition.
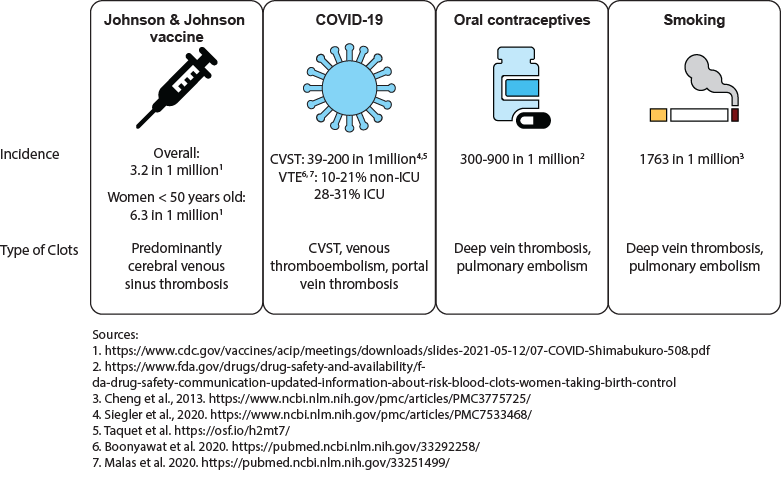
Figure 1
The risk of clotting from the Johnson & Johnson vaccine compared with the risk of clotting from COVID-19 infection, oral contraceptives and tobacco smoking.
Conclusions
Based on data from patients who developed CVST and other clots in combination with low platelet counts after the AstraZeneca vaccine, this unusual condition appears to be similar to heparin-induced thrombocytopenia. As such, it is recommended that patients be treated with non-heparin anticoagulants, potentially along with intravenous immune globulin to inhibit platelet activation. It is still unclear how the vaccine might be causing this condition and if it is something specific to the adenovirus delivery method or vaccine production methods. It remains to be seen whether the CDC and FDA will make age-based recommendations for the use of the J&J vaccine, similar to the recommendation by European regulators for the AstraZeneca vaccine. With COVID-19 infection rates still extremely high in the U.S. and globally, the J&J vaccine is still an important component to controlling the pandemic. Characteristics of the J&J vaccine, including its stability at standard refrigerator and freezer temperatures, and its single-dose regimen, make it especially ideal for reading certain populations, such as people who are homebound, experiencing homelessness or incarcerated. According to a model presented by CDC scientists, if the 10 million J&J doses sitting on shelves were deployed, over the next six months we could expect to see 26-45 cases of the clotting disorder, and 600-1,400 fewer COVID-19 related deaths.
View all COVID-19 updates
View all FLARE updates