Update in Sedation and Analgesia Management in COVID-19 ARDS
The FLARE Four
- The COVID-19 pandemic has resulted in the widespread use of deep sedation in the ICU, and there is some data that sedation practices have departed from pre-COVID-19 era recommendations
- Prior to COVID-19, robust evidence demonstrated the benefit of avoiding benzodiazepines, minimizing sedation and using protocolized vent weaning practices
- The burden on critical care services has led to downstream effects on sedation strategies and medication choices. The use of higher total doses of all sedative agents, including benzodiazepines, has been described in critically ill COVID-19 patients
- Targeting less overall sedation is associated with improved outcomes in critically ill mechanically ventilated patients, including mortality and duration of mechanical ventilation. These same principles should be applied to sedation management in COVID-19
Subscribe to the latest updates from FLARE Advances in Motion
Many people are asking…Are we doing sedation right in COVID-19?
The SARS-CoV-2 pandemic has led to a significant increase in the number of patients suffering with respiratory failure necessitating mechanical ventilation (MV). Concomitantly, there has been an increase in the use of sedative and opioid medications during MV. The scale and severity of the pandemic, along with concerns regarding ventilator synchrony and patient harm, have created significant challenges in the management of analgesia and sedation. This was previously covered on May 2, 2020.
In this FLARE, Matthew F. Mart, MD, Christina Boncyk, MD, Brenda T Pun, DNP, RN, Rafael Badenes, MD, PhD, Pratik P. Pandharipande, MD, MSCI, and E. Wesley Ely, MD, MPH, of Vanderbilt University Medical Center, provide a brief review of the state of knowledge regarding best practices for sedation and analgesia in patients receiving MV and an update on literature to date specific to COVID-19.
Sedation and Analgesia Management in ARDS in the Pre-COVID-19 Era
Patients with moderate to severe acute respiratory distress syndrome (ARDS) often require analgesia and sedation to facilitate comfort and ventilator tolerance during MV. The approach to analgesia and sedation in mechanically ventilated critically ill patients has changed dramatically in the last 30 years, with current guidelines (1) centered upon the use of analgesia-based light sedation strategies with close monitoring using validated bedside assessment tools such as the Richmond Agitation-Sedation Scale (RASS) (2) or the Sedation Assessment Scale (SAS) (3). These recommendations were based on work done by Shehabi et al. and others, which has demonstrated a strong association between deep sedation during intensive care unit (ICU) admission with an increased risk of longer MV as well as both in-hospital and 180-day mortality (Figure 1) (4), a finding demonstrated to be independent of ARDS severity in a related cohort (5). Daily interruptions in sedation (6) and the conduct of spontaneous breathing trials (SBTs) (7) reduce the overall duration of mechanical ventilation. When combined, paired SATs/SBTs are further associated with a reduction in mortality (Figure 2) (8). While these landmark studies were not specifically limited to patients with ARDS, they did account for one-quarter to one-half of patients enrolled in these trials (9).
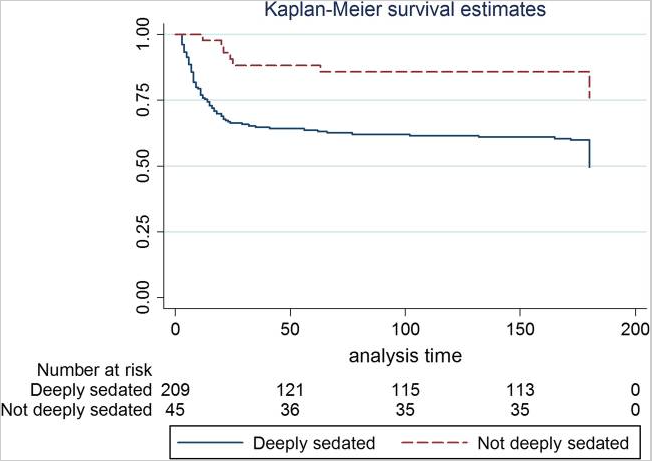
Figure 1
Kaplan-Meier survival curve of patients who were deeply sedated versus those who were not deeply sedated (i.e. light sedation). (Shehabi Y, et al. AJRCCM. 2012;186(8):724-31).
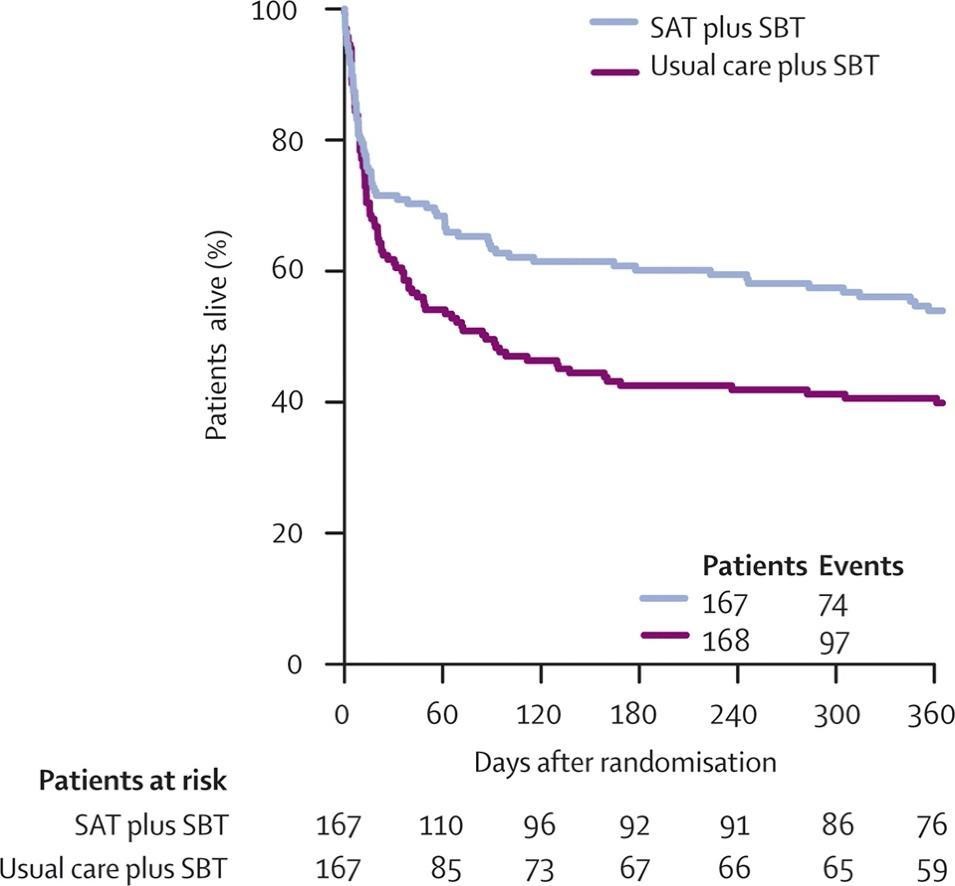
Figure 2
Kaplan-Meier curve demonstrating improved survival with the use of paired SATs & SBTs (Girard TD, et al. Lancet 2008;371(9607):126-34).
Patients with ARDS receiving MV require a multimodal treatment strategy, including the use of low-tidal volume ventilation and conservative fluid management (10, 11). Deep sedation with chemical paralysis has been used for patients with severe hypoxemia, ventilator dyssynchrony and to facilitate lung-protective low tidal volume ventilation in ARDS (12). The ACURASYS trial compared placebo versus early neuromuscular blockade in patients with early severe ARDS and found a 9% absolute reduction in 90-day mortality. Notably, patients in both study arms received similar cumulative doses of sedatives and analgesic agents to provide deep sedation. In contrast, other studies in patients with ARDS have demonstrated no harm when delivering lung-protective ventilation whilst targeting light sedation (13, 14). In the ROSE trial, a larger, follow-up study of early neuromuscular blockade in moderate to severe ARDS, there was no difference in mortality between patients managed with early deep sedation and paralysis versus light sedation, which stands in contrast to the earlier ACURASYS study (15). Notably, deep sedation can actually worsen ventilator synchrony due to ineffective triggering and entrainment/reverse triggering (16, 17). Taken together, these findings suggest that many, if not most, patients with ARDS can be managed without deep sedation. Patients should therefore be treated using a strategy that prioritizes analgesia with opioids to help pain and the ventilatory drive (18), with subsequent sedative agents used only when necessary and titrated to the lightest level of sedation possible. Levels of sedation should be monitored frequently and routinely, and all efforts should be made to limit the overall exposure to sedation.
GABAergic agents such as benzodiazepines and propofol have traditionally been used to provide targeted sedation in the ICU. In the last two decades, dexmedetomidine, a central α2-adrenoreceptor agonist, has been increasingly used for sedation in the ICU as well (19), though when used in isolation, it does not cause the same level of deep sedation as benzodiazepines and propofol. Dexmedetomidine (20, 21) and propofol (22, 23) have both been studied against benzodiazepines and found to be superior to midazolam and lorazepam with regards to ICU outcomes. Current guidelines, therefore, recommend the use of non-benzodiazepine sedation agents when needed (1), since continuous benzodiazepines infusions have been associated with significant morbidity, including being a risk factor for the development of delirium (24), a risk factor for mortality and long-term cognitive impairment (25-27). The PRODEX (22) and the recently completed MENDS2 (28) studies compared propofol versus dexmedetomidine for sedation in critically ill patients and found that outcomes were similar, including for those with sepsis. Similarly, the SPICE-III study compared dexmedetomidine versus usual care (including the use of propofol, midazolam or both per clinician discretion) in critically ill adults receiving MV and found no difference in 90-day mortality (29). Notably, there were more adverse events in the dexmedetomidine group, but this data was not collected systemically or adjudicated and was reported at the discretion of site investigators. Based on these studies, targeting light sedation with either propofol or dexmedetomidine while employing best sedation and delirium practices is recommended.
Sedation Management in the Era of COVID-19
The burden of the COVID-19 pandemic on critical care services has led to downstream effects on sedation strategies and medication choices. Impacted by concerns regarding medication shortages (necessitating the substitution of agents less frequently used prior to COVID-19) and staff limitations, some have advocated for deeper sedation. This call has been more prominent due to early speculation that patients need increased sedation due to high respiratory drives, which may limit the tolerability of low tidal volumes (30, 31). The use of higher total doses of all sedative agents, including benzodiazepines, has been described in critically ill COVID-19 patients (32). In a recently published large, multinational observational study of over 2,000 critically ill COVID-19 patients from 14 countries, deep sedation was common and prolonged (33). In this cohort, mechanically ventilated patients had a median RASS score of -4 [IQR, -5 to-3], and during the 21 day study period, the median number of days alive and free of coma or delirium was only 5 days [IQR, 0.0 to 14.0]. Additionally, approximately two-thirds of patients received benzodiazepines for a median of 7.0 days [4.0 to 12.0] (Figure 3). Consistent with prior research, mechanical ventilation, the use of restraints and benzodiazepines were all associated with a higher risk of delirium the following day (Figure 4). Despite the significant prior literature describing the risks of deep, continuous sedation and benzodiazepine use in other critically ill patients, such practices have become extremely common in critically ill and mechanically ventilated COVID-19 patients. In part, this may be due to the understandable desire to minimize vent dyssynchrony and maximize lung protection, particularly in the early phases of critical illness. However, these findings have potentially significant downstream consequences, including longer duration of mechanical ventilation (6, 7), delirium (24), increased morbidity and mortality (4) and long-term sequelae of the post-intensive care syndrome (PICS), such as cognitive impairment (26). Utilization of evidence-based protocols that include light sedation and awakening and breathing trials in critically ill COVID-19 patients is associated with a greater number of ventilator-free days as compared to non-protocolized care (34), highlighting the potential risks.
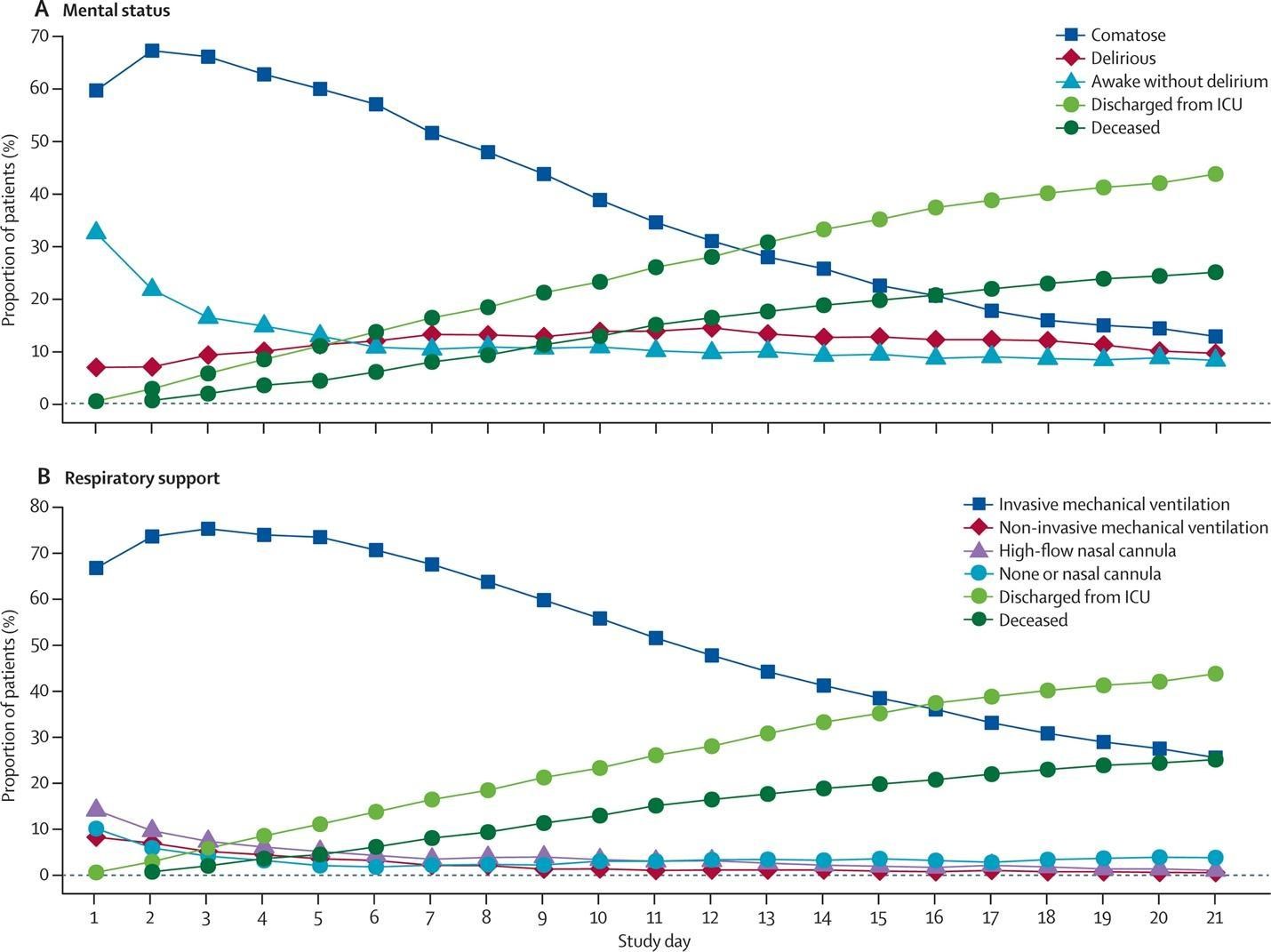
Figure 3
Level of sedation, respiratory support, mortality and ICU discharge in COVID-19 patients. (Pun BT, et al. Lancet Resp Med 2021).
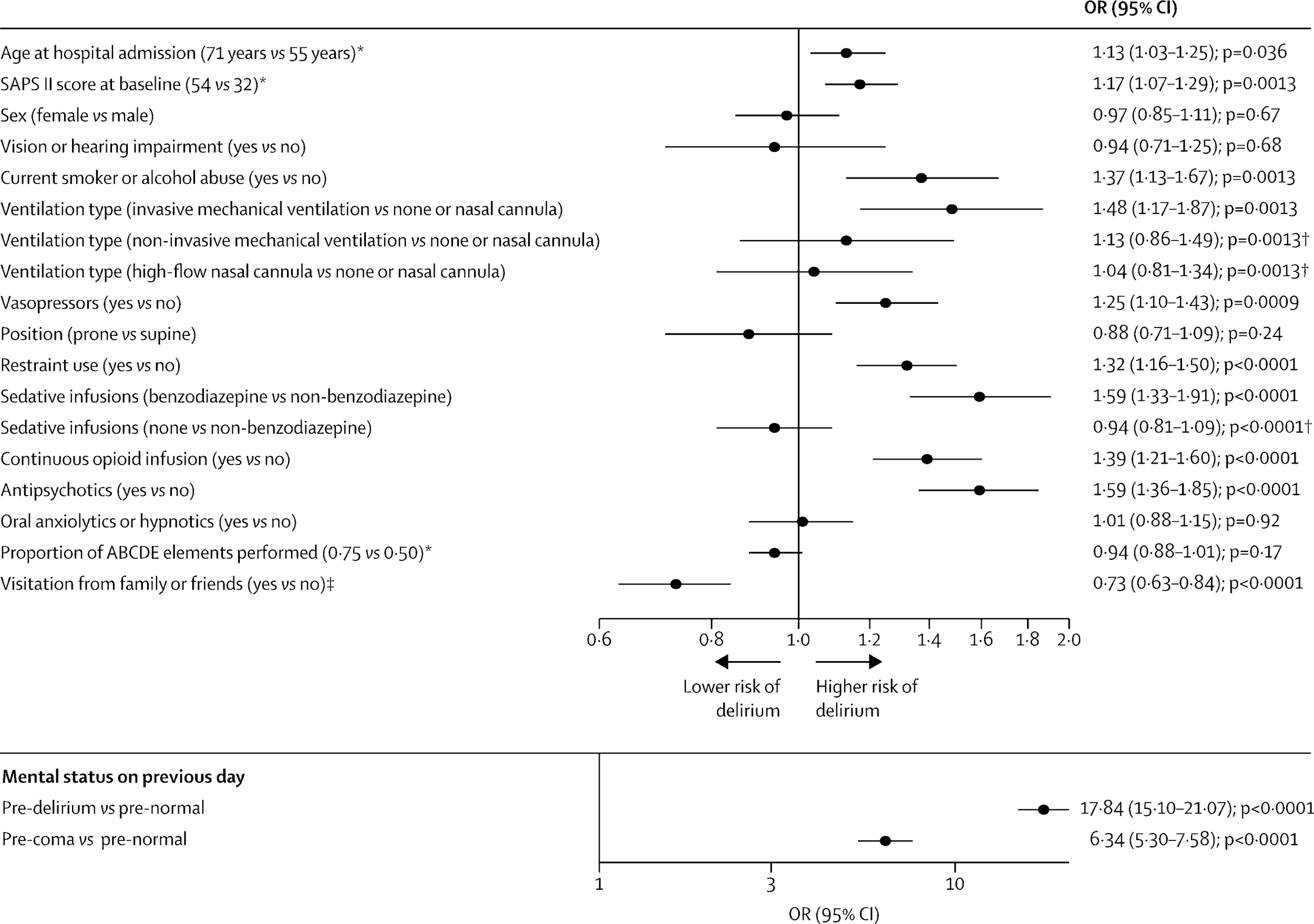
Figure 4
Forest plot of daily probability of delirium by major risk factor (Pun BT, et al. Lancet Resp Med 2021).
Limited guidance exists from either societal guidelines or randomized clinical trials to address sedation management in COVID-19 specifically. However, the predominant histopathological pattern of COVID-19 lung injury is that of diffuse alveolar damage, the hallmark pathology of ARDS (35). Additionally, the patterns of gas exchange and respiratory compliance seen in COVID-19 ARDS are consistent with those in prior ARDS cohorts (36, 37). Given the lack of substantial evidence that COVID-19 ARDS differs qualitatively from that of "classical ARDS," sedation management in critically ill COVID-19 patients should mirror the existing evidence-based guidelines (1). Careful attention to the core principles of supportive care in the ICU is necessary for the optimal care of all ARDS patients, including those with COVID-19. These foundational elements include the routine assessment and management of pain, combined spontaneous awakening and breathing trials, careful monitoring of the level of consciousness and an analgesia-first light sedation strategy that avoids of benzodiazepines, delirium assessment and management, early mobility and family engagement, all of which form the basis of the Society of Critical Care Medicine's ICU Liberation ABCDEF Bundle (Table 1).
Table 1
Components of the ABCDEF Bundle; SAT: Spontaneous awakening trial; SBT: Spontaneous breathing trial (Pun BT, et al. Crit care med. 2019;47(1):3-14)The COVID-19 pandemic has introduced several additional difficulties in the implementation and performance of the ABCDEF bundle, including shifts in critical care capacity and personnel, reduced bedside presence by staff due to the number of patients, shortages of personal protective equipment and drugs, and prolonged durations of MV due to the severity of respiratory failure. Each of these barriers, and several others, have led to increased and prolonged sedation use, potentially leading to a cascade of effects on patient outcomes. While there are multiple barriers to bundle performance with COVID-19, daily evaluation of sedation use and strategic planning is vital to adapting these important care practices to new challenges (38). Increasing ABCDEF bundle performance is important as it is associated with substantial improvements in mortality, mechanical ventilation use, ICU readmission rates, coma and delirium, and discharge to a facility (39). Clinical care ultimately occurs at the bedside, and so all clinicians should use their prudential judgment in their treatment choices. We would advocate, however, that the clinician should call upon the best evidence available when faced with a choice in sedation and analgesia management in a critically ill patient with COVID-19 ARDS. By all accounts, the evidence supports using the approach recommended by the current Clinical Guidelines on Pain, Agitation, Delirium, Immobility and Sleep Disturbances in the Adult Patients in the ICU (1) and the ABCDEF bundle.
Ongoing Questions
Despite significant evidence regarding the best practices for sedation management in respiratory failure, substantial lingering questions remain. The best sedation approach in unique clinical situations increasingly encountered during the pandemic, prone position ventilation or extracorporeal membrane oxygenation (ECMO), remains unclear. In patients with ARDS, prone positioning has been shown to reduce mortality in patients with moderate to severe ARDS (40). While continuous neuromuscular blockade is not a necessary component of prone position ventilation, the vast majority of patients receive sedation and neuromuscular blocking agents (40, 41). To date, no prospective, randomized trials have specifically evaluated sedation strategies during prone positioning.
Like prone positioning, ECMO has been increasingly deployed during the COVID-19 pandemic given the severity and global scale of disease (42). While the majority of patients on ECMO have also received mechanical ventilation and thus have likely been managed with both analgesic and sedative agents, the presence of large intravascular cannulae creates important considerations for the intensivist. Catastrophic ECMO malfunction from inadvertent dislodgment of vascular access is a serious concern, such that traditionally many patients have been managed with deep sedation, often concurrently with neuromuscular blockade. Such an approach may also facilitate "lung rest" or an "ultra-protective" approach to mechanical ventilation (with tidal volumes <4 mL/kg predicted body weight), one approach advocated in patients on ECMO (43), though not yet proven. In contrast, strategies that prioritize early weaning of sedation, spontaneous breathing and mobilization on ECMO, have also been proposed (44, 45). Limited data exists currently to serve clinicians in this emerging area of critical care. Given that the majority of patients who are cannulated for ECMO have severe ARDS, the findings of the ACURASYS and ROSE trials serve as guideposts but do not definitely answer the question of sedation management in this clinical area. Frequent assessments of clinical status and patient physiology, built upon the tenets of evidence-based supportive care, currently serve as the best guide for the intensivist at the bedside caring for COVID-19 patients receiving these advanced therapies. Even in these situations, however, clinicians should evaluate the ongoing sedation needs daily, and use it at the lightest level and for the shortest duration possible.
Conclusions
The historic scale and severity of the COVID-19 pandemic have brought the challenges of sedation and analgesia during mechanical ventilation and critical illness into stark relief, highlighted by increased use of deep sedation and benzodiazepines. Over the last two decades, significant evidence has demonstrated that targeting less overall sedation is associated with improved outcomes in critically ill, mechanically ventilated patients, including mortality and duration of mechanical ventilation. This can be challenging in the setting of ARDS, particularly early in the illness, when the risk of sedation must be balanced with the need for lung protection. Nevertheless, a substantial body of literature prior to COVID-19 indicates that minimizing sedation will drastically improve outcomes. These same principles apply to sedation management in COVID-19 as they do in other etiologies of ARDS. While unique circumstances and clinical judgment remain important to the clinician at the bedside, reliance on the evidence-based guidelines for sedation management remains a vital component of care in optimal critical care in COVID-19.
References
1. Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Critical Care Medicine. 2018;46(9):e825-e73.
2. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. American Journal of Respiratory and Critical Care Medicine. 2002;166(10):1338-44.
3. Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Critical Care Medicine. 1999;27(7):1325-9.
4. Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. American Journal of Respiratory and Critical Care Medicine. 2012;186(8):724-31.
5. Tanaka LM, Azevedo LC, Park M, Schettino G, Nassar AP, Réa-Neto A, et al. Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Crit Care. 2014;18(4):R156.
6. Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. The New England Journal of Medicine. 2000;342(20):1471-7.
7. Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. The New England Journal of Medicine. 1996;335(25):1864-9.
8. Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet (London, England). 2008;371(9607):126-34.
9. Shah FA, Girard TD, Yende S. Limiting sedation for patients with acute respiratory distress syndrome - time to wake up. Curr Opin Crit Care. 2017;23(1):45-51.
10. ARDSNet. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. The New England Journal of Medicine. 2000;342(18):1301-8.
11. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. The New England Journal of Medicine. 2006;354(24):2564-75.
12. Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. The New England Journal of Medicine. 2010;363(12):1107-16.
13. Kahn JM, Andersson L, Karir V, Polissar NL, Neff MJ, Rubenfeld GD. Low tidal volume ventilation does not increase sedation use in patients with acute lung injury. Critical Care Medicine. 2005;33(4):766-71.
14. Mehta S, Cook DJ, Skrobik Y, Muscedere J, Martin CM, Stewart TE, et al. A ventilator strategy combining low tidal volume ventilation, recruitment maneuvers, and high positive end-expiratory pressure does not increase sedative, opioid, or neuromuscular blocker use in adults with acute respiratory distress syndrome and may improve patient comfort. Ann Intensive Care. 2014;4:33.
15. Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, et al. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. The New England Journal of Medicine. 2019;380(21):1997-2008.
16. Akoumianaki E, Lyazidi A, Rey N, Matamis D, Perez-Martinez N, Giraud R, et al. Mechanical ventilation-induced reverse-triggered breaths: a frequently unrecognized form of neuromechanical coupling. Chest. 2013;143(4):927-38.
17. Vaschetto R, Cammarota G, Colombo D, Longhini F, Grossi F, Giovanniello A, et al. Effects of propofol on patient-ventilator synchrony and interaction during pressure support ventilation and neurally adjusted ventilatory assist. Critical Care Medicine. 2014;42(1):74-82.
18. Devlin JW, Roberts RJ. Pharmacology of commonly used analgesics and sedatives in the ICU: benzodiazepines, propofol, and opioids. Anesthesiol Clin. 2011;29(4):567-85.
19. Keating GM. Dexmedetomidine: A Review of Its Use for Sedation in the Intensive Care Setting. Drugs. 2015;75(10):1119-30.
20. Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644-53.
21. Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489-99.
22. Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151-60.
23. Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell-Bright S, Levitt J, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Critical Care Medicine. 2006;34(5):1326-32.
24. Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21-6.
25. Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr., et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-62.
26. Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. The New England Journal of Medicine. 2013;369(14):1306-16.
27. Lonardo NW, Mone MC, Nirula R, Kimball EJ, Ludwig K, Zhou X, et al. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patients. American Journal of Respiratory and Critical Care Medicine. 2014;189(11):1383-94.
28. Hughes CG, Mailloux PT, Devlin JW, Swan JT, Sanders RD, Anzueto A, et al. Dexmedetomidine or Propofol for Sedation in Mechanically Ventilated Adults with Sepsis. The New England Journal of Medicine. 2021.
29. Shehabi Y, Howe BD, Bellomo R, Arabi YM, Bailey M, Bass FE, et al. Early Sedation with Dexmedetomidine in Critically Ill Patients. The New England Journal of Medicine. 2019;380(26):2506-17.
30. Payen JF, Chanques G, Futier E, Velly L, Jaber S, Constantin JM. Sedation for critically ill patients with COVID-19: Which specificities? One size does not fit all. Anaesth Crit Care Pain Med. 2020;39(3):341-3.
31. Hanidziar D, Bittner EA. Sedation of Mechanically Ventilated COVID-19 Patients: Challenges and Special Considerations. Anesth Analg. 2020;131(1):e40-e1.
32. Kapp CM, Zaeh S, Niedermeyer S, Punjabi NM, Siddharthan T, Damarla M. The Use of Analgesia and Sedation in Mechanically Ventilated Patients With COVID-19 Acute Respiratory Distress Syndrome. Anesth Analg. 2020;131(4):e198-e200.
33. Pun BT, Badenes R, Heras La Calle G, Orun OM, Chen W, Raman R, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. The Lancet Respiratory Medicine. 2021.
34. Janz DR, Mackey S, Patel N, Saccoccia BP, St Romain M, Busack B, et al. Critically Ill Adults With Coronavirus Disease 2019 in New Orleans and Care With an Evidence-Based Protocol. Chest. 2021;159(1):196-204.
35. Borczuk AC, Salvatore SP, Seshan SV, Patel SS, Bussel JB, Mostyka M, et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33(11):2156-68.
36. Fan E, Beitler JR, Brochard L, Calfee CS, Ferguson ND, Slutsky AS, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8(8):816-21.
37. Panwar R, Madotto F, Laffey JG, van Haren FMP. Compliance Phenotypes in Early Acute Respiratory Distress Syndrome before the COVID-19 Pandemic. American Journal of Respiratory and Critical Care Medicine. 2020;202(9):1244-52.
38. Devlin JW, O'Neal HR, Jr., Thomas C, Barnes Daly MA, Stollings JL, Janz DR, et al. Strategies to Optimize ICU Liberation (A to F) Bundle Performance in Critically Ill Adults With Coronavirus Disease 2019. Crit Care Explor. 2020;2(6):e0139.
39. Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, et al. Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Critical Care Medicine. 2019;47(1):3-14.
40. Guérin C, Reignier J, Richard J-C, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. The New England Journal of Medicine. 2013;368(23):2159-68.
41. Guérin C, Beuret P, Constantin JM, Bellani G, Garcia-Olivares P, Roca O, et al. A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study. Intensive Care Med. 2018;44(1):22-37.
42. Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071-8.
43. Bein T, Weber-Carstens S, Goldmann A, Müller T, Staudinger T, Brederlau J, et al. Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus 'conventional' protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39(5):847-56.
44. Swol J, Shekar K, Protti A, Tukacs M, Broman LM, Barrett NA, et al. Extubate Before VV ECMO Decannulation or Decannulate While Remaining on the Ventilator? The EuroELSO 2019 Weaning Survey. Asaio j. 2020.
45. Lehr CJ, Zaas DW, Cheifetz IM, Turner DA. Ambulatory extracorporeal membrane oxygenation as a bridge to lung transplantation: walking while waiting. Chest. 2015;147(5):1213-8.
View all FLARE content
Learn more about research in the Division of Pulmonary and Critical Care Medicine