COVID-19 Vaccine Candidates
The FLARE Four
- Five vaccine candidates have been demonstrated in phase 1 and 2 trials to induce an immune response that compares favorably to that produced by natural SARS-CoV-2 infection, and they are in or entering phase 3 trials in the U.S.
- Duration of vaccine-induced immunity to SARS-CoV-2, safety information and detailed phase 3 efficacy data remain unreported. Two candidates have announced unpublished results from the first interim analysis of phase 3 trials demonstrating roughly 95% efficacy
- Successful interim analysis of a phase 3 trial and emergency use authorization will be rapidly followed by the start of vaccine distribution in the coming weeks to months. Vaccine availability is expected to be limited in the initial phase of distribution
- Important ethical challenges will arise around (1) continuing placebo arms in ongoing vaccine trials once there is an approved vaccine and (2) equitable vaccine distribution in the setting of initial scarcity
The effort to produce and distribute a SARS-CoV-2 vaccine has made remarkable progress. Currently there are over 180 candidate vaccines against SARS-CoV-2 in development worldwide (Krammer 2020). Pfizer and Moderna have both announced promising results from the first interim analysis of their phase 3 studies, raising hope that a vaccine will be ready before the end of 2020.
Subscribe to the latest updates from FLARE Advances in Motion
These hopeful predictions come with concern that political pressure and demands for rapid development will lead to approval of a vaccine candidate before necessary safety assessments can be completed. To protect against this, on October 6, 2020, the Food and Drug Administration (FDA) updated their guidance document for vaccine developers with stricter recommendations. Due to the overwhelming need, it is expected that once clinical trial data are available, the FDA will rapidly move towards an emergency use authorization based on this guidance.
In this FLARE, we review the current state of the vaccine development effort with a focus on candidate approaches expected to post results in the United States in the coming weeks to months.
What is Operation Warp Speed?
Normally the process of taking a drug or vaccine from the laboratory to the clinic takes several years. Preclinical studies identify a target and vaccine strategy. Phase 1 studies aim to determine the highest tolerable dose in a relatively small number of healthy participants, and can take several months. Phase 2 studies enroll up to several hundred participants and look at short-term side effects and the immune response to the vaccine. This typically takes several months or up to two years, and these trials are usually not large enough to determine efficacy. Finally, phase 3 studies enroll thousands of volunteers to study the efficacy of the vaccine compared to a placebo or control group. Phase 3 trials typically take one to four years, which can identify long-term or rare side effects.
Operation Warp Speed (OWS), introduced in April 2020, aims to produce and deliver 300 million doses of safe and effective vaccine, with the initial doses available by the end of 2020. It seeks to condense a process that normally takes 15 years or more into 10 to 18 months (Krammer 2020). Part of the acceleration in the timeline comes from running steps in the traditional development timeline simultaneously, such as beginning industrial-scale manufacturing prior to vaccine approval. Additionally, under OWS, resources such as clinical trial sites and the data safety monitoring board (DSMB), which monitors safety concerns as well as early signs of success, are shared among the participating study sponsors. An advantage of a shared DSMB is that if a potential safety issue appears in one trial, the DSMB can look out for that issue across all trials. Moderna, Johnson & Johnson and AstraZeneca have all received funding for vaccine research and development through OWS and share the DSMB. Pfizer has organized an independent DSMB and has not received money for vaccine development. (Pfizer has secured a $1.95 billion distribution deal with the U.S. government, which is contingent on vaccine approval.)
Vaccine Strategies
We covered strategies used to produce a vaccine against SARS-CoV-2 in a prior FLARE on April 24. Of the various approaches, five have begun (or will soon begin) phase 3 studies in the United States. All of the current candidates utilize the SARS-CoV-2 spike protein as the antigen. The SARS-CoV-2 spike protein is very similar to the spike protein on the closely related virus SARS-CoV, which causes SARS. Because of this, vaccine development efforts benefitted from years of work on SARS and crucial development time was saved (Krammer 2020). The spike protein is a trimeric protein, displayed on the SARS-CoV-2 envelope, that engages cell-surface receptors (Wu et al. 2020) in order to facilitate viral entry into host cells (Chivukula et al. 2020). Once bound to a cell surface receptor, the spike protein undergoes a conformational change. Mutations in critical proline residues stabilize the protein in its pre-fusion conformation and this is the form which is most often used in vaccine candidates.
RNA Vaccines
RNA-based designs have generated interest because they may be integrated into generic manufacturing platforms (Liu 2019). The ease and speed of making genetic constructs mean that such platforms offer the potential for rapid design, construction and manufacture of vaccines against epidemic or emerging diseases such as SARS-CoV-2 once the genetic sequence is known. This technology is relatively new, however, and as such few RNA-based vaccines have made it to clinical trials, and none has ever been approved for use.
The basic approach to an mRNA vaccine is as follows. An mRNA molecule that encodes a viral protein of interest is created in vitro. Naked mRNA is quickly degraded in vivo, so the vaccine mRNA is incorporated into some type of delivery system that facilitates cellular uptake, such as lipid nanoparticles. Once intracellular, the mRNA is translated by the host cell into an antigenic protein that induces an immune response. While both DNA-based and RNA-based vaccines have been proposed, vaccines composed of RNA offer some advantages. mRNA is non-infectious and non-integrating (meaning it does not integrate into the host genome—a key concern surrounding DNA vaccines) (Pardi et al. 2018). mRNA-based vaccines may also be easier to manufacture, as they are created by chemical synthesis and do not need to be grown in and purified from cells. On the other hand, these vaccines must be kept at fairly cold temperatures to ensure stability, which produces logistical challenges for distribution. The Moderna vaccine needs to be stored at -20 degrees Celsius, while the Pfizer vaccine requires -70 C.
Viral Vector-Based Techniques
A viral vector-based approach is one in which a distinct virus is engineered to express the SARS-CoV-2 spike protein or another SARS-CoV-2 antigen. This approach has the advantage of the strong immunogenicity of live attenuated vaccines with the safety of subunit vaccines (i.e., no live SARS-CoV-2). However, immunity to the vector itself (rather than the spike protein) may limit effectiveness. This means that if the carrier virus is attacked by the immune system, it may not survive long enough to induce immunity to the viral protein. For this reason, candidate vaccines often use viral vectors, such as chimpanzee viruses, that people are unlikely to have previously developed immunity to.
Recombinant Protein-Based Techniques
Recombinant protein-based techniques typically focus on the synthesis of the viral spike protein or its subunits. This approach is well-validated and has been used for a number of currently-approved vaccines. Potential concerns include limited immunogenicity (though adjuvants—which are additives such as aluminum salts or oils that induce immunogenicity—can be added to enhance the immune response) and more challenging production, particularly on the massive scale that would be needed to match global demand.
What is the FDA looking for before they issue an EUA?
- In June, the FDA issued guidance that stated they would expect a vaccine to result in 50% fewer cases of symptomatic COVID-19 in vaccinated participants compared with placebo
- In October, the FDA added that they would want to see follow-up safety data for a median of at least two months after completion of the full vaccination regimen—i.e., that at least half of participants would need to complete two months of follow up after the second dose. This time frame is consistent with the time over which complications would be expected to develop
- In addition, the FDA asked for documentation of at least five cases of severe COVID-19 in the placebo group in order to better assess the risk of vaccine-associated enhanced respiratory disease (VAERD)
VAERD refers to worsened respiratory disease following vaccination and viral infection, compared to what would normally be seen with viral infection alone, and first became a concern in the 1960s during vaccine trials for respiratory syncytial virus (RSV) where vaccinated infants showed worsened disease and qualitatively different inflammation compared to non-vaccinated infants (Bottazzi et al. 2020). There is conflicting evidence of VAERD in reports from vaccine development efforts against SARS and MERS (Krammer 2020). A particularly severe form of adverse immune reaction is antibody-dependent enhancement as seen in Dengue infection. We have previously reviewed concerns antibody-dependent enhancement (ADE) in the April 18 FLARE.
- Vaccine makers have also been required to include in their protocols strategies for continuing clinical trials after EUA and for handling the loss of follow-up information
The FDA plans to convene a public Vaccines and Related Biological Products Advisory Committee meeting to discuss the available safety and efficacy data of a particular vaccine, prior to issuing an EUA, with publicly available briefing documents on the FDA webpage no later than two full business days before the day of the advisory meeting.
Phase 3 trials in the US
- Four vaccine candidates (Moderna, BioNTech/Pfizer, Johnson & Johnson and AstraZeneca) have begun phase 3 trials in the U.S., and a fifth (Novavax) is expected to launch later in November (Novavax has already started phase 3 trials in the U.K.)
- Two of the candidates (Moderna and BioNTech/Pfizer) are mRNA vaccines. Currently, no commercially available vaccine uses this technology, and prior to COVID-19 it has not been tested in large-scale human trials (Abbasi 2020)
- Johnson & Johnson and AstraZeneca use a more traditional viral vector strategy (in this case, non-replicating adenovirus expressing the SARS-CoV-2 spike protein). Johnson & Johnson's Ad26 adenovirus is the same vector used in its approved vaccine for Ebola, while AstraZeneca ChAdOx1 is a vector that causes common colds in chimpanzees
- Novavax uses recombinant full-length spike protein that forms a rosette formation they refer to as a "nanoparticle," with an adjuvant for increased immunogenicity (Krammer 2020)
All of the candidates target the SARS-CoV-2 spike protein, which is the protein that binds to the host cell and mediates viral entry into the cell (Huang et al. 2020). AstraZeneca's candidate uses the wild-type spike protein, while the other candidates include mutations that stabilize the spike protein in its pre-fusion conformation. Studies examining other coronaviruses, such as Middle East respiratory syndrome coronavirus (MERS-CoV) have suggested that spike proteins in the pre-fusion conformation elicit a stronger antibody response (Pallesen et al. 2017).
In an unusual move, pharmaceutical companies have released their full clinical trial study protocols (typically these documents are not shared until after clinical trials are completed), allowing the public and outside experts to evaluate the study designs. Of the four candidates in Phase 3 trials in the U.S., only Johnson & Johnson has set its primary efficacy objective as the prevention of moderate or severe COVID-19; the others have a primary efficacy objective of preventing any COVID-19, no matter the severity, which has been criticized by some experts. Those trials do have secondary efficacy objectives that seek to evaluate the vaccine candidate in preventing severe COVID-19. Additionally, while these trials are enrolling tens of thousands of participants, all exclude children and adolescents, immunocompromised patients, pregnant or breastfeeding women. There are concerns that the studies are not adequately designed to evaluate whether the vaccine candidate offers significant protection to elderly participants, who are at high risk of severe COVID-19 (Doshi 2020).
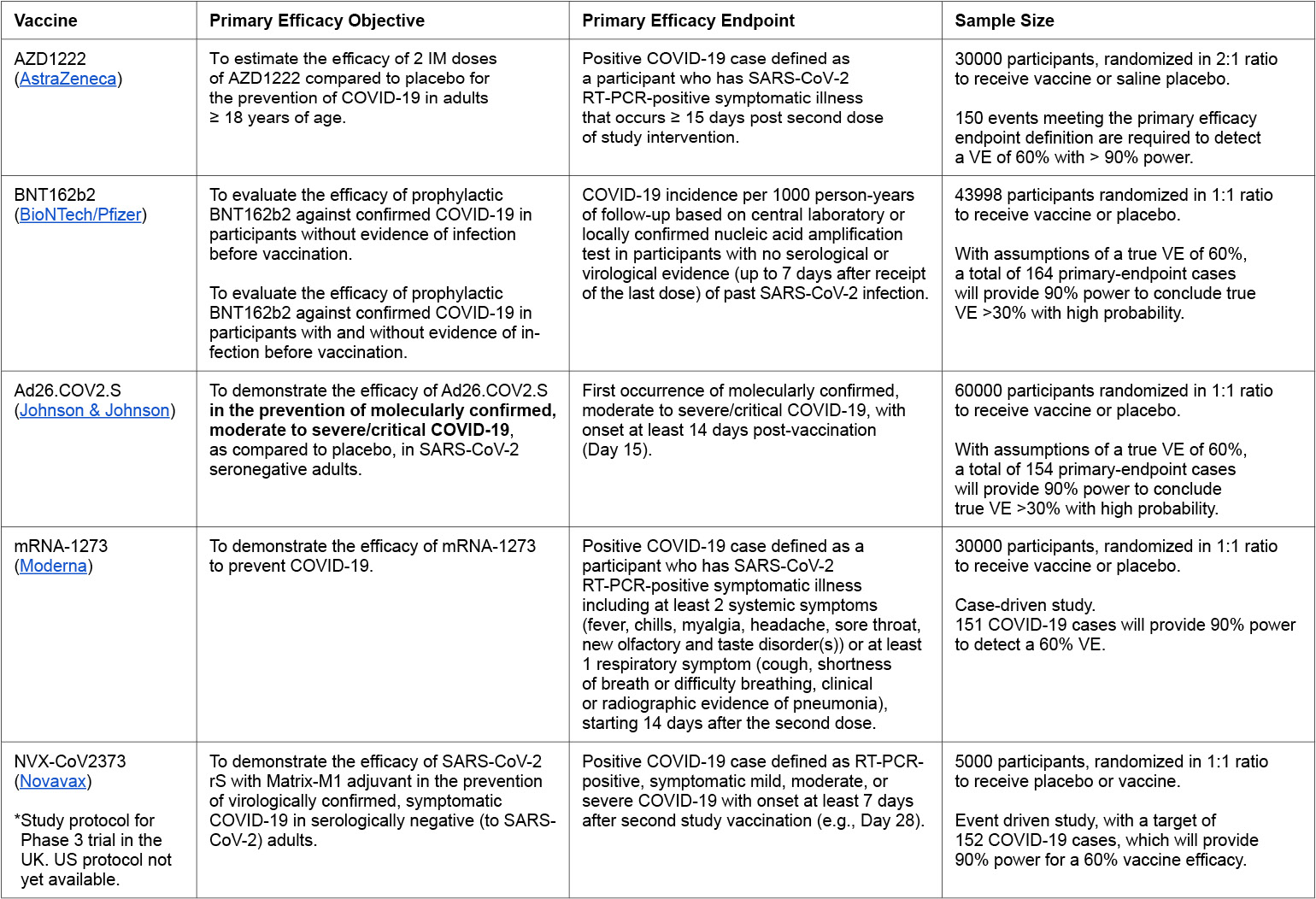
Table 1:
VE = vaccine efficacy
AstraZeneca - Adenovirus
AstraZeneca's vaccine, AZD1222, uses a non-replicating chimpanzee adenovirus platform to express the wild-type version of the spike protein (it does not contain stabilizing mutations). Virus-based vaccine platforms have the risk that the immune system will clear the virus before it can infect host cells to produce the antigen. The use of a chimpanzee adenovirus is designed to reduce the likelihood of vector clearance as it is unlikely the patient's immune system would have encountered this virus before.
Results from the phase 1 and 2 trials in adults aged 18 to 55 show that the vaccine was generally tolerated, with no serious adverse events, and mild side effects that could be reduced with acetaminophen (Folegatti et al. 2020). In phase 1 and 2 trials, they tested the response following a single vaccination versus a booster vaccine after 28 days, with the MenACWY meningococcal vaccine as a comparator vaccine, to ensure that local or systemic reactions to vaccination would not result in unblinding of participants. Increases in trimeric SARS CoV-2 spike protein, measured by standardized total IgG ELISA, were found in participants receiving both a single dose and a booster dose which persisted for 56 days and were comparable to levels seen in convalescent plasma samples collected from symptomatic patients. Virus neutralization assays showed comparable neutralizing titers as compared to convalescent plasma from known COVID-19 patients and health care workers. AstraZeneca has not yet released data on how adults over 65 years old respond to their vaccine.
AstraZeneca paused its trial on September 6 for a safety review, after a participant reportedly developed symptoms of transverse myelitis, or inflammation of the spinal cord. On Oct 23 the FDA authorized the restart of U.S. trials. To the extent that Pfizer and Moderna have not reported any potential safety concerns this finding could advantage those approaches. Johnson and Johnson also reported sporadic, potential adverse events. As such, the AstraZeneca and Johnson and Johnson trials will now be monitored to see if these events occur in more patients which could terminate the studies.
BioNTech/Pfizer – mRNA
While Pfizer has secured a $1.95 billion distribution deal with the U.S. government to supply up to 100 million doses of its vaccine for free, it notably has not taken funds for vaccine development nor does it share resources for coordinating and monitoring the clinical trials. Additionally, Pfizer has announced it will use its own vaccine distributor, rather than McKesson Corporation, which has an existing contract with the CDC to support vaccine distribution. A possible reason for using their own distribution system is the need to monitor and keep the vaccine at -70C (-94F).
Pfizer tested two candidates, BNT162b1 and BNT162b2, in phase 1 and 2 trials, and ultimately proceeded with BNT162b2 for phase 3 trials due to its milder reactogenicity profile. BNT162b1 encodes for the SARS-CoV-2 receptor binding domain, while BNT162b2 encodes the full-length SARS-CoV-2 spike protein, with 2 mutations to lock it in the prefusion conformation. Results from phase 1 and 2 trials show that both BNT162b1 and BNT162b2 produced greater amounts of neutralizing antibodies compared to human COVID-19 convalescent sera in both young and older adults (Walsh et al. 2020).
Pfizer made major headlines when they announced on November 9 that interim analysis of their phase 3 trial showed that the BNT162b2 vaccine was more than 90% effective in preventing COVID-19. Pfizer has subsequently announced that, to date, they have seen 170 cases of COVID-19 in their phase 3 trial—162 in the placebo arm and eight in the treatment arm. There were 10 cases of severe COVID-19 seen in the trial—nine of them in the placebo group. Pfizer has estimated that they will need to collect data from 164 patients to demonstrate the required 50% efficacy. Prior to applying for an EUA, they will need to collect at least two months of safety data from at least half of the participants in the trial before it can apply for an EUA; this could be as early as the end of November. Pfizer currently projects it could produce up to 50 million vaccine doses globally by the end of 2020.
Johnson & Johnson - Adenovirus
Unlike other vaccine candidates in phase 3 trials in the U.S. that require two doses administered three to four weeks apart, Johnson & Johnson's non-replicating adenovirus-based design only requires a single injection, though they are also studying a two-dose regimen as a backup. Johnson & Johnson's virus platform, AdVac®, is a non-replicating adenovirus and has already been tested and approved for use in Ebola vaccines. The adenovirus expresses the stabilized pre-fusion spike protein of SARS-CoV-2. The vaccine would be compatible with standard vaccine distribution channels, as it is anticipated to be stable for two years at -20°C and at least three months at 2° to 8°C.
Phase 1 and 2 trials in healthy adults (age 18-55) and the elderly (≥65) tested two doses of the vaccine, with two injections administered eight weeks apart. Interim results looking at antibody responses four weeks after the first dose (Sadoff et al. 2020) showed robust levels of spike protein-specific antibodies, measured by ELISA. In addition, they showed neutralizing antibody titers, measured by a wild-type virus neutralization assay, in both age groups. These antibodies were at levels comparable to a human convalescent serum panel.
Johnson & Johnson launched its phase 3 trials in September, aiming to enroll 60,000 participants worldwide. It was paused on Oct 12, after a participant suffered a stroke following vaccination. An investigation could not identify a clear cause for the stroke, nor evidence that the stroke was triggered by the vaccine, and the trial resumed on Oct 23.
Moderna - mRNA
Moderna announced it had completed the enrollment of 30,000 participants for its phase 3 trial (NCT04470427) on Oct 22. High-risk groups (people over 65, or with conditions that place them at high risk of severe COVID-19) comprise 42% of trial participants, and 37% of participants are from communities of color.
The Moderna candidate, mRNA-1273, encodes the SARS-CoV-2 spike glycoprotein, with modifications to stabilize it in its pre-fusion conformation, and is encapsulated in a lipid nanoparticle. Results from phase 1 trials showed that 25ug and 100ug dosing was generally well tolerated in healthy volunteers ranging from age 18 to over 71 (Jackson et al. 2020; Anderson et al. 2020), and immune responses were generally similar across age groups.
Prior to the COVID-19 pandemic, Moderna had already been testing their mRNA platform in phase 1 and phase 2 clinical trials against viruses such as cytomegalovirus, Zika, Chikungunya, as well as in the treatment of tumors and melanoma. On Nov 16, Moderna announced that an interim analysis of their phase 3 trial showed a 94.5% vaccine efficacy. This analysis included 95 cases of COVID-19 (five cases in the vaccine group, 90 cases in the placebo group), including 11 cases that were deemed "severe," based on the study protocol definitions. Additionally, 15 of the cases were in older adults, and 20 were from participants who identified as being from diverse communities. Moderna needs to reach 151 COVID-19 cases to complete the trial and anticipates that, with the surge in COVID-19 cases in the U.S., it should reach that endpoint in the coming weeks. Moderna's CEO Stéphane Bancel has stated he anticipates Moderna will apply for an EUA by Thanksgiving, with 20 million doses ready to ship in the U.S. by the end of 2020. A major advantage of the Moderna vaccine over the Pfizer vaccine is that it can remain stable at 2-8°C (a standard refrigerator temperature) for 30 days, or at -20°C (a standard freezer temperature) for up to six months. Additionally, it can remain stable at room temperature for up to 12 hours. This means that the vaccine can be shipped via established distribution methods, and can be stored in readily available freezers and refrigerators.
Novavax - Recombinant Nanoparticle
Novavax's vaccine is a recombinant nanoparticle vaccine consisting of recombinant SARS-CoV-2 spike glycoprotein stabilized in the prefusion conformation, which is administered with the saponin-based Matrix-M1(™) adjuvant. The adjuvant is standard and the expression system used to produce the protein is similar to the one used for the production of Flublok, an FDA-approved influenza vaccine by Sanofi Pasteur.
Phase 1 and 2 trials tested two doses of the vaccine, with and without the Matrix-M1 adjuvant, in healthy adults aged 18 to 59 (Keech et al. 2020). Reactogenicity was absent or mild in the majority of participants across all groups. Overall, subjects who received the vaccine with Matrix-M1 adjuvant showed antibody titers comparable to human convalescent plasma samples (collected from patients who were asymptomatic, outpatient symptomatic or hospitalized), with those receiving two doses of the vaccine showing titers comparable to plasma samples from patients hospitalized with COVID-19. The group who received the vaccine alone (no adjuvant) showed low antibody titers. Similarly, in a wild-type SARS-CoV-2 microneutralization assay, subjects receiving two doses of the vaccine with Matrix-M1 showed neutralizing antibody responses similar to the convalescent serum panel, while a single dose or two doses without Matrix-M1 showed little neutralizing activity.
Novavax has an ongoing phase 3 clinical trial in the U.K. and expects to complete enrollment for that study by the end of November. Novavax received "Fast Track Designation" from the FDA on Nov 9, meaning regulatory review will be expedited, and anticipates beginning phase 3 trials in the U.S. and Mexico by the end of November.
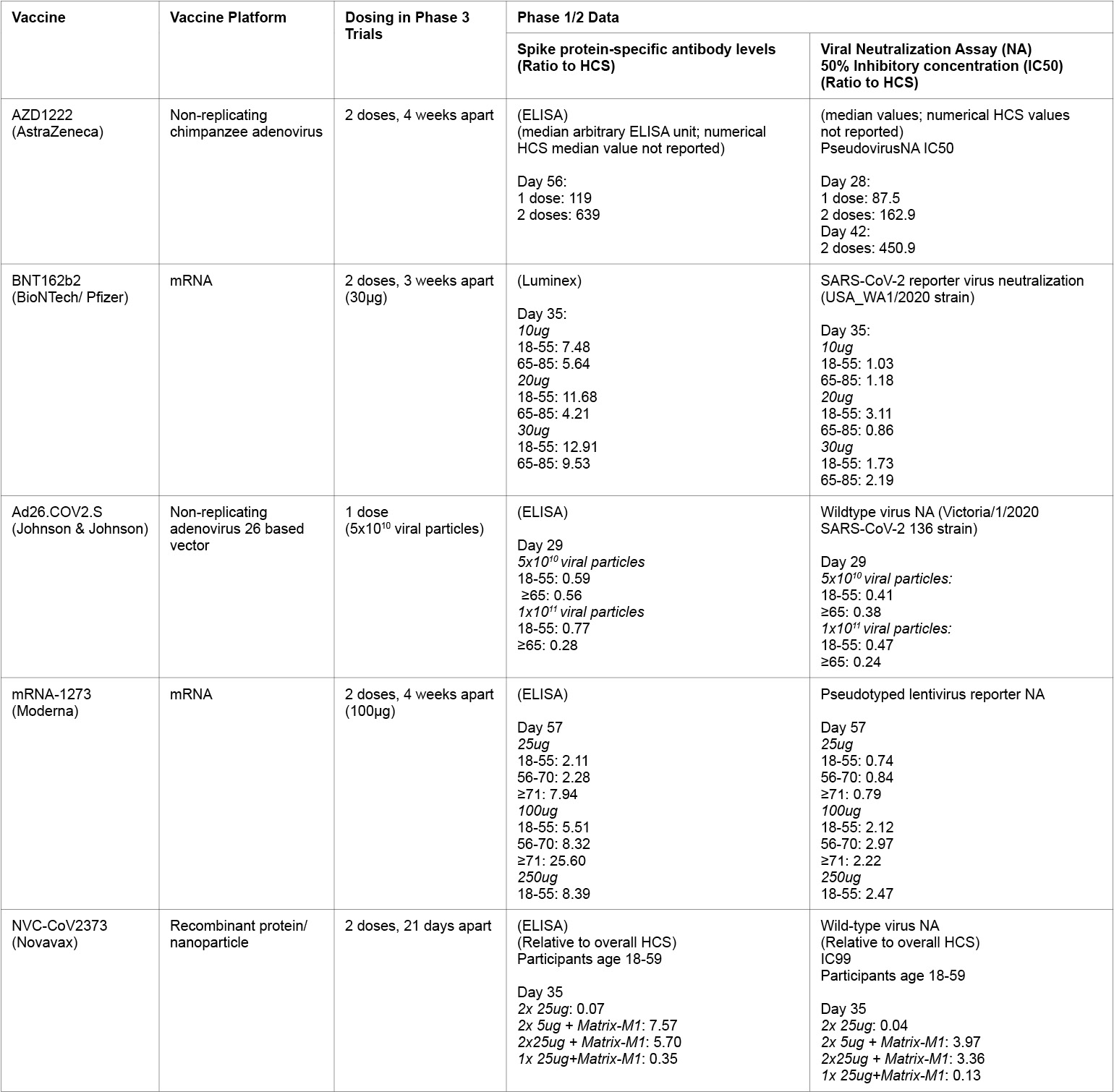
Table 2:
HCS = Human convalescent serum panel
Vaccine Distribution
Given the worldwide scale of the pandemic, vaccine distribution will pose logistical and ethical challenges. In the United States, the CDC has released an interim operational guidebook that covers strategies and objectives for the rollout of a nationwide vaccination program. Assuming the Pfizer and Moderna efficacy, safety and production data are all accurate, there will likely be something on the order of 70 million vaccine doses available by the end of the year (enough to vaccinate 35 million people, given the two-dose regimen). This number is clearly less than the expected need and CDC anticipates a phased vaccination program (Figure 1) with initial doses going to health care workers, other essential workers and those at high risk for severe COVID-19. It is expected that the federal government will allocate initial doses to local jurisdictions (state, territory and tribal governments) with initial allocations reflecting vaccine availability, vaccine distribution capability and size of the critical population within a jurisdiction. In order to ensure equitable distribution of the vaccine, the CDC Advisory Committee on Immunization Practices, the National Institutes of Health, the National Academies of Sciences, Engineering and Medicine are all involved in determining initial populations of focus.
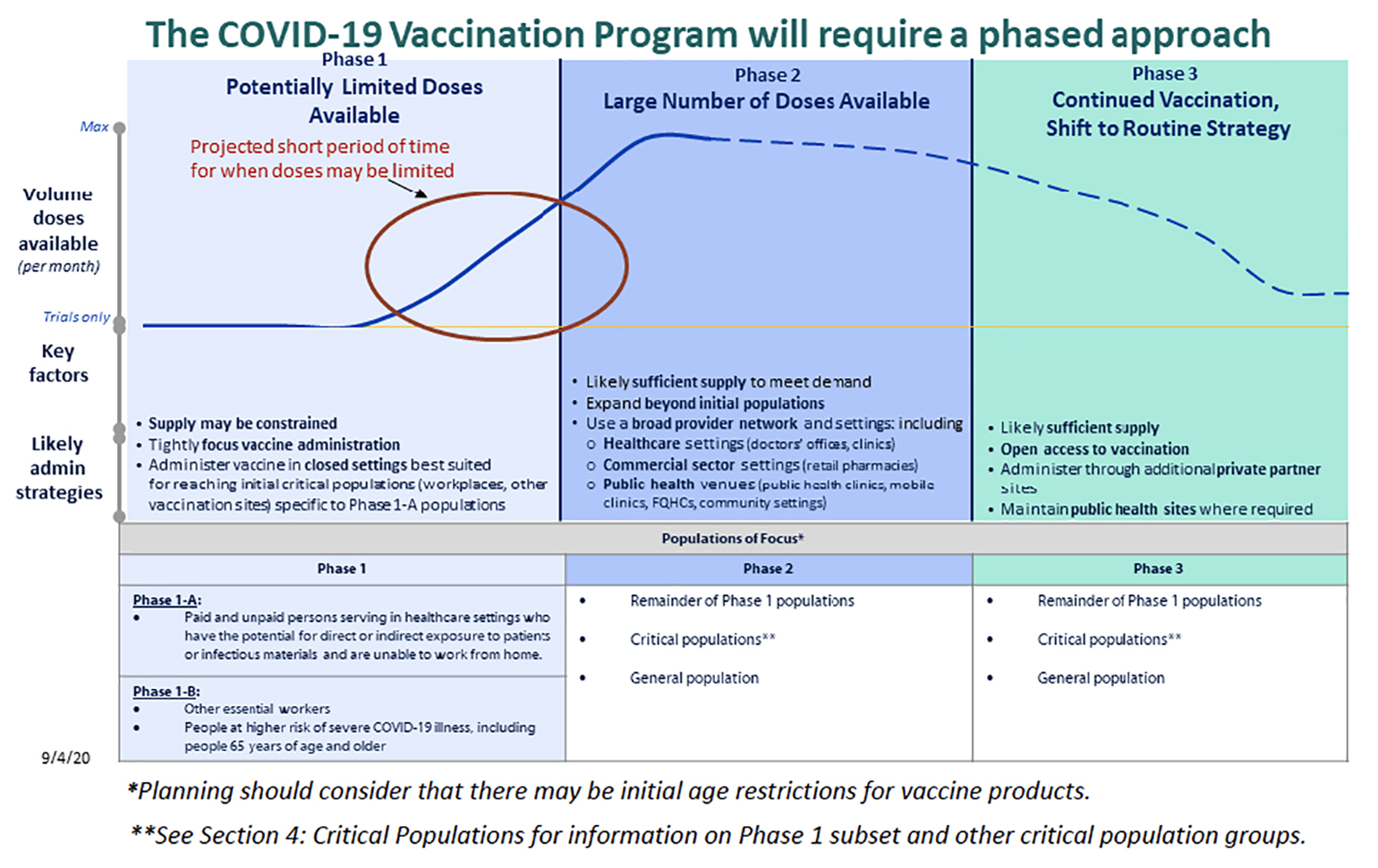
Figure 1
Phased distribution of a COVID-19 vaccine. Phase I likely is limited to frontline healthcare works (Phase 1A), followed by other essential workers and people at higher risk for severe illness (Phase IB). From the COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. (Photo credit: Centers for Disease Control and Prevention)
Conclusion
The vaccine development effort has made remarkable progress in a very short time, in part, due to the recent dramatic rise in COVID-19 throughout the world. Based on company press releases, it appears that two candidates (Pfizer and Moderna) have already demonstrated efficacy. It is reasonable to hope that the first vaccine doses will be available by the end of this year. Even after one or multiple of these vaccine candidates prove safe and effective, the road to mass vaccination remains filled with challenges. In the event that multiple candidates receive EUAs, given that most of the candidates require two injections spaced 21 or 28 days apart, one challenge will be to ensure that patients receive the correct second dose at the correct time point. An EUA of one vaccine could also raise ethical questions in ongoing trials, both for the approved vaccine as well as for other candidates. Participants will likely want to know whether they received the placebo, and may drop out in order to receive the approved vaccine, which will make it more difficult to gather long-term data on the safety and efficacy. The FDA has stated that it will require companies applying for an EUA to include strategies to address this potential problem. Finally, the demand will exceed the supply of vaccines. A major effort is thus underway to plan for equitable distribution of the initially limited supply. To date, only the board outlines of this program are publicly available.
References
Abbasi, Jennifer. 2020. "COVID-19 and mRNA Vaccines-First Large Test for a New Approach." JAMA: The Journal of the American Medical Association .324 (12): 1125–27.
Anderson, Evan J., Nadine G. Rouphael, Alicia T. Widge, Lisa A. Jackson, Paul C. Roberts, Mamodikoe Makhene, James D. Chappell, et al. 2020. "Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults." The New England Journal of Medicine. September. https://doi.org/10.1056/NEJMoa2028436.
Bottazzi, Maria Elena, Ulrich Strych, Peter J. Hotez, and David B. Corry. 2020. "Coronavirus Vaccine-Associated Lung Immunopathology-What Is the Significance?" Microbes and Infection/ Institut Pasteur. 22 (9): 403–4.
Chivukula, Raghu R., Jason H. Maley, David M. Dudzinski, Kathryn Hibbert, and C. Corey Hardin. 2020. "Evidence-Based Management of the Critically Ill Adult With SARS-CoV-2 Infection." Journal of Intensive Care Medicine. October, 885066620969132.
Doshi, Peter. 2020. "Will Covid-19 Vaccines Save Lives? Current Trials Aren't Designed to Tell Us." BMJ. 371 (October): m4037.
Folegatti, Pedro M., Katie J. Ewer, Parvinder K. Aley, Brian Angus, Stephan Becker, Sandra Belij-Rammerstorfer, Duncan Bellamy, et al. 2020. "Safety and Immunogenicity of the ChAdOx1 nCoV-19 Vaccine against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial." The Lancet. 396 (10249): 467–78.
Huang, Yuan, Chan Yang, Xin-Feng Xu, Wei Xu, and Shu-Wen Liu. 2020. "Structural and Functional Properties of SARS-CoV-2 Spike Protein: Potential Antivirus Drug Development for COVID-19." Acta Pharmacologica Sinica. 41 (9): 1141–49.
Jackson, Lisa A., Evan J. Anderson, Nadine G. Rouphael, Paul C. Roberts, Mamodikoe Makhene, Rhea N. Coler, Michele P. McCullough, et al. 2020. "An mRNA Vaccine against SARS-CoV-2 - Preliminary Report." The New England Journal of Medicine. July. https://doi.org/10.1056/NEJMoa2022483.
Keech, Cheryl, Gary Albert, Iksung Cho, Andreana Robertson, Patricia Reed, Susan Neal, Joyce S. Plested, et al. 2020. "Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine." The New England Journal of Medicine. September. https://doi.org/10.1056/NEJMoa2026920.
Krammer, Florian. 2020. "SARS-CoV-2 Vaccines in Development." Nature. 586 (7830): 516–27.
Liu, Margaret A. 2019. "A Comparison of Plasmid DNA and mRNA as Vaccine Technologies." Vaccines. 7 (2). https://doi.org/10.3390/vaccines7020037.
Pallesen, Jesper, Nianshuang Wang, Kizzmekia S. Corbett, Daniel Wrapp, Robert N. Kirchdoerfer, Hannah L. Turner, Christopher A. Cottrell, et al. 2017. "Immunogenicity and Structures of a Rationally Designed Prefusion MERS-CoV Spike Antigen." Proceedings of the National Academy of Sciences of the United States of America. 114 (35): E7348–57.
Pardi, Norbert, Michael J. Hogan, Frederick W. Porter, and Drew Weissman. 2018. "mRNA Vaccines - a New Era in Vaccinology." Nature Reviews. Drug Discovery. 17 (4): 261–79.
Riel, Debby van, and Emmie de Wit. 2020. "Next-Generation Vaccine Platforms for COVID-19." Nature Materials. 19 (8): 810–12.
Sadoff, Jerry, Mathieu Le Gars, Georgi Shukarev, Dirk Heerwegh, Carla Truyers, Anna Marit de Groot, Jeroen Stoop, et al. 2020. "Safety and Immunogenicity of the Ad26.COV2.S COVID-19 Vaccine Candidate: Interim Results of a Phase 1/2a, Double-Blind, Randomized, Placebo-Controlled Trial." Infectious Diseases (except HIV/AIDS). medRxiv.
The Economist. 2020. "Understanding SARS-CoV-2 and the Drugs That Might Lessen Its Power." The Economist. March 12, 2020. https://www.economist.com/briefing/2020/03/12/understanding-sars-cov-2-and-the-drugs-that-might-lessen-its-power.
Walsh, Edward E., Robert W. Frenck Jr, Ann R. Falsey, Nicholas Kitchin, Judith Absalon, Alejandra Gurtman, Stephen Lockhart, et al. 2020. "Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates." The New England Journal of Medicine. October. https://doi.org/10.1056/NEJMoa2027906.
Wu, Fan, Su Zhao, Bin Yu, Yan-Mei Chen, Wen Wang, Zhi-Gang Song, Yi Hu, et al. 2020. "A New Coronavirus Associated with Human Respiratory Disease in China." Nature. 579 (7798): 265–69.
View all COVID-19 updates
Learn more about the Division of Pulmonary and Critical Care Medicine