The Burden of Cognitive Dysfunction in COVID-19
The FLARE Four
- Delirium, coma and long-term cognitive dysfunction are distressingly common in critical illness and can be understood as part of a common syndrome—acute brain dysfunction
- Acute brain dysfunction is a concern in COVID-19 because the circumstances of the pandemic (staffing shortages, heavy sedation, lack of family interaction) led to difficulty in implementing many practices known to reduce acute brain dysfunction
- As a result, delirium incidence in critical COVID-19 has been reported to be high compared to pre-pandemic levels. This may be expected to lead to an increased number of patients suffering post-intensive care syndrome after their ICU discharge
- Evidence-based interventions developed prior to the COVID-19 pandemic, such as the ABCDEF bundle, should be implemented to the greatest extent possible in order to decrease the incidence of acute brain dysfunction in COVID-19
Many people are asking…what is the burden of cognitive dysfunction in COVID-19?
Subscribe to the latest updates from FLARE Advances in Motion
Introduction
Acute brain dysfunction manifesting as delirium and coma is a highly prevalent form of organ dysfunction in critically ill patients, particularly those receiving mechanical ventilation. Among critically ill patients, it is associated with increased morbidity and long-term sequelae comprising physical, cognitive and emotional symptoms, referred to as post-intensive care syndrome (PICS). PICS significantly impacts the quality of life for both survivors and their family members for years following hospital discharge. Years of research targeting delirium prevention and treatment have included both pharmacologic intervention and non-pharmacologic strategies. To date, the most successful strategies for decreasing the prevalence of intensive care unit (ICU) delirium include targeting light sedation, reducing benzodiazepine exposure and mobilizing patients. The challenges presented by the COVID-19 pandemic, however, have strained clinicians and hospital systems, frequently resulting in a deviation from these best practices. (Challenges with sedation practice in COVID-19 were reviewed in FLARE on May 2, 2020.) Consequently, critically ill COVID-19 patients have shown a profound burden of acute brain dysfunction. Given the burden of acute brain dysfunction and the additional burden posed by isolation common among COVID-19 survivors, these patients are at an exaggerated risk for the long-term development of PICS-related cognitive dysfunction or acquired dementia.
This FLARE is written by Christina Boncyk, MD; Matthew F. Mart, MD; Brenda T Pun, DNP, RN; Rafael Badenes, MD, PhD; Pratik P. Pandharipande, MD, MSCI; E. Wesley Ely, MD, MPH, of Vanderbilt University Medical Center.
What Do We Know About Delirium in the ICU?
There are several known risk factors for delirium that include age, impaired baseline cognitive function, severity of illness, mechanical ventilation, medication administration (particularly benzodiazepines and increased sedation) and sepsis, among others. Due to the numerous physiologic and pharmacologic derangements associated with critical illness, delirium is common in the ICU with older studies describing a prevalence of over 80% in mechanically ventilated patients. Even though delirium is extremely prevalent, up to 3 out of 4 cases will be undetected if not systematically assessed using validated delirium-monitoring instruments because of the hypoactive motoric predominance of symptoms at the bedside. Early identification of delirium serves as an alert to team members to consider contributing factors and alter patient care to mitigate the duration of delirium. This is important as increased duration of delirium is associated with worse patient outcomes, specifically mortality and cognitive impairment among survivors. We now know that the use of multimodal and interprofessional management approaches that require care team buy-in and coordination have dramatically lowered the prevalence and duration of delirium.
Proven Strategies to Reduce the Burden of Brain Dysfunction in the ICU
Within the ICU, the ABCDEF (A2F) bundle has been the most widely adopted and studied strategy to reduce the burden of brain dysfunction. Implementation has been shown to improve patient outcomes, specifically decreasing delirium duration, when implemented across hospitals and care settings. The tenets of the A2F bundle are drawn from over 30 original investigations and are designed to optimize patient safety. They include:
- Assessing and treating pain
- Both awakening and breathing trials
- Choice of appropriate sedation
- Delirium monitoring and management
- Early mobility and exercise
- Family engagement and empowerment
The A2F bundle is supported by the Society of Critical Care Medicine as a pillar of the ICU Liberation Initiative and has been shown to be an independent predictor of reductions in delirium, coma, and duration of mechanical ventilation and ICU stay in a dose-dependent manner (i.e., higher compliance yields incrementally better results) across multiple settings (Figure 1).
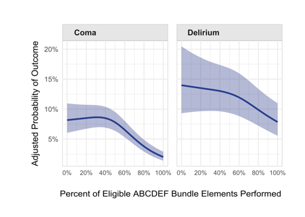
Figure 1
The association between proportional performance of the ABCDEF bundle and delirium and coma (Pun et al, Crit Care Med, 2019).
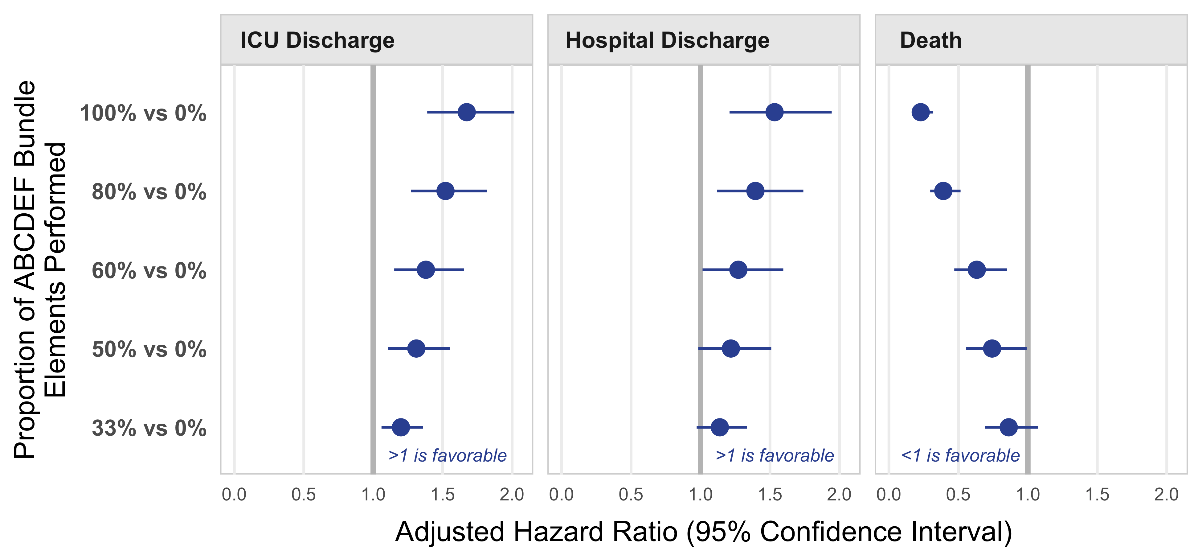
Figure 2
The association between percent performance of the ABCDEF bundle and ICU discharge, hospital discharge, and death (Pun et al, Crit Care Med, 2019).
While each component has been shown to be effective individually, the impact of the A2F bundle is strongest when it is performed together, as each component influences the strength of all others (Figure 2). As stated by others, the whole is greater than the sum of the parts. For example, daily awakening trials and spontaneous breathing trials are both independently shown to decrease time to extubation and improve patient outcomes; however, they are most effective when paired and performed together—activities that require coordination and planning between physicians, nurses and respiratory therapists.
Acute Brain Dysfunction in COVID-19 Patients
Delirium on presentation or, more generally, "abnormal neurologic examinations" at admission have been described in patients with COVID-19. To investigate the prevalence of delirium within this population, Pun and colleagues performed a large multinational, cohort investigation of over 2,000 critically ill COVID-19 patients. Delirium and coma were measured prospectively using validated instruments to describe the overwhelming burden of acute brain dysfunction within this population. While a recently completed multicenter study that adhered to A2F bundle guidelines reported a delirium prevalence of less than 50% (marking an example of the scope of delirium reduction seen in recent years), Pun and colleagues found a resurgence of delirium—up to 80% of critically ill COVID-19 patients developed delirium and coma. Over the 21-day study period, the median number of days alive and without coma and delirium was only 5 [0-14]. Additionally, A2F bundle components were incorporated into practice in only about 25% of eligible days. Benzodiazepine usage was associated with a higher risk of brain dysfunction while family visitation was associated with decreased risk of delirium. These results are concerning, but echo reports of COVID-19 patient care deviating away from multicomponent care bundles and receiving higher doses of sedative and analgesics than patients without COVID-19.
Challenges to Diagnosis and Management of Acute Brain Dysfunction in COVID-19
The COVID-19 pandemic has strained health care providers across hospital systems and intensive care units. As the number of patients has grown exponentially, so have the number of COVID-19-related ICU admissions. Management of these admissions has necessitated rapid adaptation of care teams to expand critical care coverage outside of usual ICU locations and utilization of health care personnel not otherwise caring for the critically ill. Previously avoided sedatives (e.g., benzodiazepines) are frequently used due to concerns about medication shortages and the perceived need for deep sedation. Additionally, infection control concerns frequently result in limited interaction with health care providers and limited or complete visitation restrictions for family members. As a result, delirium prevention bundles previously implemented as standard of care are frequently sidelined. While these surge and workflow adaptations have been successful in helping to accommodate and provide care to the increasing number of patients, they directly or indirectly may be contributing to acute brain dysfunction among COVID-19 patients. Thoughtful and deliberate strategies to navigate limited personnel, medications or equipment may help to improve bundle compliance and patient care delivery.
ABCDEF Care Bundle and COVID-19 Specific Challenges to Implementation
A: Assess, prevent & manage pain
Bundle Intervention: Assess pain with CPOT or BPS scales, NRS if patient able to self-report; use of regional analgesia and non-opioid adjuncts; analgesia-based sedation techniques with fentanyl
COVID-19-Specific Barriers to Implementation: Isolation policies limiting pain assessments; medication shortages
COVID-19 Specific Barrier Guidance: Covid-19 Sedation Summary Guideline
B: Both SAT & SBT
Bundle Intervention: Daily linked SAT and SBT; interprofessional team coordination of care
COVID-19-Specific Barriers to Implementation: Provider reluctance; staffing shortages; inability to implement coordination at large scale
COVID-19 Specific Barrier Guidance:
C: Choice of sedation
Bundle Intervention: Targeted light sedation when able; avoidance of benzodiazepines; dexmedetomidine if at high risk for delirium or weaning mechanical ventilation
COVID-19-Specific Barriers to Implementation: Ventilator dyssynchrony; medication shortages
COVID-19 Specific Barrier Guidance:
- Am I Using Really High Doses of Sedation for My Ventilated COVID-19 Patients?
- Strategies for Weaning Analgesia and Sedation
D: Delirium monitoring & management
Bundle Intervention: Routine assessments with CAM-ICU, CAM-ICU-7, or ICDSC; non-pharmacologic interventions including sleep hygiene
COVID-19-Specific Barriers to Implementation: Isolation policies limiting pain assessments; staffing shortage
COVID-19 Specific Barrier Guidance: Understanding and Addressing the Long-term Outcomes of COVID-19 ICU Survivors
E: Early mobility & exercise
Bundle Intervention: Physical and occupational therapy assessment; coordinate activity with SAT or periods of no sedation; passive range of motion
COVID-19-Specific Barriers to Implementation: Isolation policies limiting staff and family engagement; provider reluctance; staffing shortages
COVID-19 Specific Barrier Guidance: Creative Approaches for Early Mobility in Patients
F: Family engagement & empowerment
Bundle Intervention: Unit orientation; education; emotional and verbal support; empowerment; participation in multidisciplinary rounds
COVID-19-Specific Barriers to Implementation: Isolation policies limiting visitation; family reluctance or fear; illness in family members
COVID-19 Specific Barrier Guidance:
Familial Engagement: A Particular Challenge
A powerful component uniquely affected by this infectious disease pandemic has been family engagement and empowerment. Lack of family support (even via virtual aids) is associated with an increased burden of acute brain dysfunction. Delirium and associated isolation, even after recovery of acute illness, contribute to patient loneliness, stress, anxiety, depression and post-traumatic stress syndromes—especially in patients with baseline cognitive impairment and dementia. COVID-19 patients have also described significant depressive symptoms on self-reported scales, although the true prevalence is limited without formal clinical diagnostic assessments.
Implications of Brain Dysfunction on Survivorship and COVID-Long Haul Syndrome
Survivors of critical illness suffer from cognitive, functional and neuropsychological impairments for months to years following hospital discharge (Figure 3). This constellation of symptoms that encompass PICS frequently prevents survivors from returning to their pre-illness quality of life, independence and work. Early reports in patients surviving COVID-19 have described similar persistent symptoms affecting a multitude of organ systems, termed post-acute COVID-19, long COVID or COVID-19 long haul syndrome. Given the increasing number of critical illness survivors as a result of the COVID-19 pandemic, there is potential for creating an epidemic of patients with PICS.
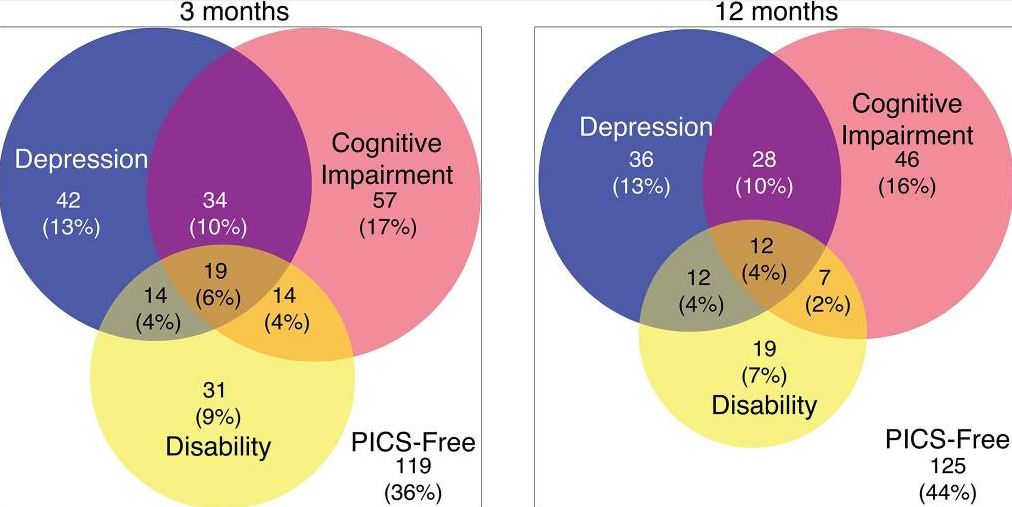
Figure 3
The co-occurrence of PICS symptoms at 3 and 12 months (Marra A, et al. Crit Care Med, 2018).
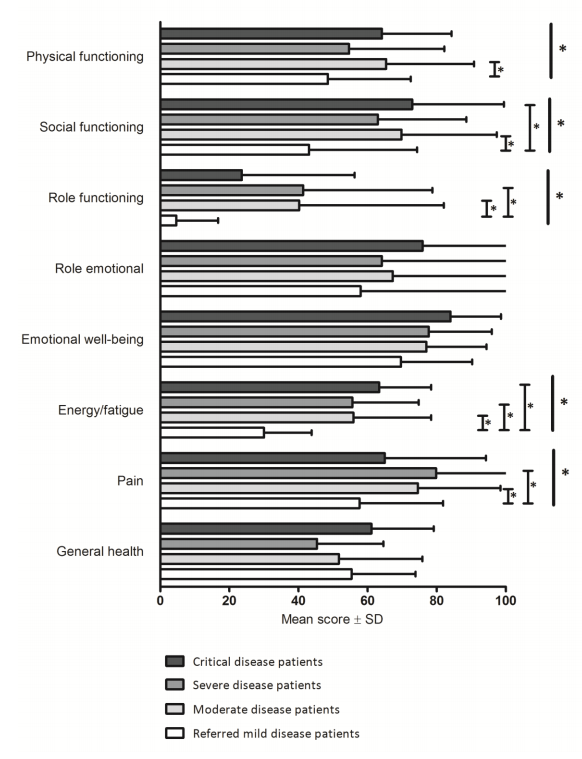
Figure 4
Heath status data from patients across the spectrum of mild to critical COVID-19 disease (van den Borst, et al., Clin Infect Dis, 2020).
Delirium is one of the strongest predictors of cognitive impairment among survivors and the high rates of brain dysfunction seen in COVID-19 patients put these patients at increased risk. Similar to the high proportion of cognitive impairment identified in previous studies of PICS in ICU survivors, early data has suggested COVID-19 survivors diagnosed with delirium demonstrate lower cognitive scores on the Telephone Interview for Cognitive Status (TICS) assessments at 4-weeks following hospital discharge. van den Borst and colleagues studied COVID-19 survivors at three months following hospital discharge and found over a third of these patients reported physical and/or cognitive difficulties, specifically in the domains of physical functioning, fatigue and quality of life—similar to results found in other ICU survivors with PICS. Notably, however, they did not find illness severity (mild, moderate or severe symptoms) to be associated with physical or cognitive status at three months (Figure 4), meaning that the incidence of these deficits was similar in mildly ill patients as in severity ill.
These findings are similar to those shown within a recent study of 238 COVID-19 survivors in Northern Italy. Patients were studied at 4-months following hospital discharge and demonstrated a significant burden of physical functional impairment (53.8%) and post-traumatic stress symptoms (17.2%). While data was collected retrospectively without the inclusion of delirium or coma diagnoses, ICU admission was not found to be independently associated with physical functional impairment, and mode of oxygen delivery (i.e., nasal cannula, mechanical ventilation) was not independently associated with post-traumatic stress symptoms. Together these studies draw into question how, or if, the severity of illness is associated with PICS symptoms within this population, but suggest a larger at-risk population. The burden of PICS symptoms among survivors and the scale of those affected is a unique challenge presented by this pandemic.
Impact on Patient Recovery
It is difficult to draw further conclusions surrounding the long-term implications of acute brain dysfunction on survivorship following COVID-19 infection as this disease has only been recognized for a little over a year. While faced with these limitations, however, there are important insights we can gain from prior coronavirus pandemic studies as well the breadth of existing literature investigating its impact on survivorship following other forms of acute respiratory distress syndrome (ARDS). ARDS is defined by a profound inflammatory response in the lung parenchyma; however, there is substantial systemic inflammation that can promote cognitive decline and neurodegenerative disease. COVID-19 infection results in an increase in these same inflammatory mediators and studies in older adults have demonstrated evidence of cognitive impairment and motor deficits following discharge. In the setting of ARDS, this is also shown to be associated with long-term cognitive impairments in up to 78% of patients at one year following hospital discharge and in about half of patients at two years.
Studies on the SARS-CoV-1 epidemic in 2003 that infected 8,000 people and killed 900 people worldwide have also focused on long-term follow-up. These studies at 1-, 2-, and up 15-year follow-up intervals have revealed a decrease in health-related quality of life metrics in survivors compared to their age-matched controls. Survivors also reported high levels of psychological distress (59%) and psychiatric disorders that persisted up to 30-months of follow-up (33%). These patients, not unlike many current COVID-19 survivors, face additional stressors related to their illness including loss of income, fear, death of friends and/or relatives, isolation and social stigma. Many of these psychological symptoms, similar to diagnosing acute brain dysfunction, can go undetected and untreated if not routinely assessed by health care providers.
Conclusions
Acute brain dysfunction is highly prevalent in COVID-19 patients. Focusing on light sedation strategies, avoidance of benzodiazepines, daily spontaneous awakening and breathing trials, family engagement, and delirium monitoring and management are key to limiting the impact of delirium and coma on long-term outcomes after COVID-19 critical illness. The impact of acute brain dysfunction on PICS symptoms and patient recovery likely qualitatively mirrors that of other ICU survivors, however, the quantity of this population is dramatically increased and may pose significant public health challenges in the future. Focusing on current best-practice techniques including multicomponent care bundles should be adhered to as much as possible to help mitigate these risks.
REFERENCES
1. Marra A, Pandharipande PP, Shotwell MS, et al. Acute Brain Dysfunction: Development and Validation of a Daily Prediction Model. Chest. 2018;154(2):293-301.
2. Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66-73.
3. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients - Validity and reliability of the Confusion Assessment Method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710.
4. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762.
5. Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39(2):371-379.
6. Myers EA, Smith DA, Allen SR, Kaplan LJ. Post-ICU syndrome: Rescuing the undiagnosed. J Am Acad Phys Assist. 2016;29(4):34-37.
7. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. The New England Journal of Medicine. 2013;369(14):1306-1316.
8. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520.
9. Girard TD, Exline MC, Carson SS, et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. The New England Journal of Medicine. 2018.
10. Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644-2653.
11. Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. The Lancet Respiratory Medicine. 2013;1(7):515-523.
12. Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489-499.
13. Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874-1882.
14. Trogrlic Z, van der Jagt M, Bakker J, et al. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Critical Care (London, England). 2015;19:157.
15. Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med. 2014;42(5):1024-1036.
16. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit Care Med. 2018.
17. Barnes-Daly MA, Phillips G, Ely EW. Improving Hospital Survival and Reducing Brain Dysfunction at Seven California Community Hospitals: Implementing PAD Guidelines Via the ABCDEF Bundle in 6,064 Patients. Crit Care Med. 2017;45(2):171-178.
18. Pun BT, Badenes R, Heras La Calle G, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. The Lancet Respiratory Medicine. 2021.
19. Spronk PE, Riekerk B, Hofhuis J, Rommes JH. Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med. 2009;35(7):1276-1280.
20. van Eijk MM, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J, Slooter AJ. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med. 2009;37(6):1881-1885.
21. Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859-864.
22. Pisani MAK, S. Y. J.; Kasl, S. V.; Murphy, T. E.; Araujo, K. L. B.; Van Ness, P. H. Days of Delirium Are Associated with 1-Year Mortality in an Older Intensive Care Unit Population. Am J Resp Crit Care Med. 2009;180(11):1092-1097.
23. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit Care Med. 2019;47(1):3-14.
24. Barnes-Daly MA, Pun BT, Harmon LA, et al. Improving Health Care for Critically Ill Patients Using an Evidence-Based Collaborative Approach to ABCDEF Bundle Dissemination and Implementation. Worldviews on Evidence-based Nursing. 2018;15(3):206-216.
25. Ely EW. The ABCDEF Bundle: Science and Philosophy of How ICU Liberation Serves Patients and Families. Crit Care Med. 2017;45(2):321-330.
26. Miller MA, Govindan S, Watson SR, Hyzy RC, Iwashyna TJ. ABCDE, but in that order? A cross-sectional survey of Michigan intensive care unit sedation, delirium, and early mobility practices. Annals of the American Thoracic Society. 2015;12(7):1066-1071.
27. Helms J, Kremer S, Merdji H, et al. Neurologic Features in Severe SARS-CoV-2 Infection. The New England Journal of Medicine. 2020;382(23):2268-2270.
28. Rebora P, Rozzini R, Bianchetti A, et al. Delirium in Patients with SARS-CoV-2 Infection: A Multicenter Study. J Am Geriatr Soc. 2020.
29. Rice TW, Janz DR. In Defense of Evidence-based Medicine for the Treatment of COVID-19 Acute Respiratory Distress Syndrome. Annals of the American Thoracic Society. 2020;17(7):787-789.
30. Janz DR, Mackey S, Patel N, et al. Critically Ill Adults With Coronavirus Disease 2019 in New Orleans and Care With an Evidence-Based Protocol. Chest. 2021;159(1):196-204.
31. Kapp CM, Zaeh S, Niedermeyer S, Punjabi NM, Siddharthan T, Damarla M. The Use of Analgesia and Sedation in Mechanically Ventilated Patients With COVID-19 Acute Respiratory Distress Syndrome. Anesth Analg. 2020;131(4):e198-e200.
32. Zhu ZP, Zhou HM, Ni YJ, Wu C, Zhang CJ, Ling XY. Can dexmedetomidine reduce atrial fibrillation after cardiac surgery? A systematic review and meta-analysis. Drug Design Development and Therapy. 2018;12:521-531.
33. Hanidziar D, Bittner EA. Sedation of Mechanically Ventilated COVID-19 Patients: Challenges and Special Considerations. Anesth Analg. 2020;131(1):e40-e41.
34. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770.
35. Emmerton D, Abdelhafiz A. Delirium in Older People with COVID-19: Clinical Scenario and Literature Review. SN Compr Clin Med. 2020:1-8.
36. Manca R, De Marco M, Venneri A. The Impact of COVID-19 Infection and Enforced Prolonged Social Isolation on Neuropsychiatric Symptoms in Older Adults With and Without Dementia: A Review. Front Psychiatry. 2020;11:585540.
37. Shrira A, Hoffman Y, Bodner E, Palgi Y. COVID-19-Related Loneliness and Psychiatric Symptoms Among Older Adults: The Buffering Role of Subjective Age. The American Journal of Geriatric Psychiatry. 2020;28(11):1200-1204.
38. Devlin JW, O'Neal HR, Jr., Thomas C, et al. Strategies to Optimize ICU Liberation (A to F) Bundle Performance in Critically Ill Adults With Coronavirus Disease 2019. Crit Care Explor. 2020;2(6):e0139.
39. Cai X, Hu X, Ekumi IO, et al. Psychological Distress and Its Correlates Among COVID-19 Survivors During Early Convalescence Across Age Groups. The American Journal of Geriatric Psychiatry. 2020;28(10):1030-1039.
40. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
41. Boutoleau-Bretonnière C, Pouclet-Courtemanche H, Gillet A, et al. The Effects of Confinement on Neuropsychiatric Symptoms in Alzheimer's Disease During the COVID-19 Crisis. Journal of Alzheimer's Disease: JAD. 2020;76(1):41-47.
42. Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. The Lancet Respiratory Medicine. 2014;2(5):369-379.
43. Marra A, Pandharipande PP, Girard TD, et al. Co-Occurrence of Post-Intensive Care Syndrome Problems Among 406 Survivors of Critical Illness. Crit Care Med. 2018;46(9):1393-1401.
44. Hatch R, Young D, Barber V, Griffiths J, Harrison DA, Watkinson P. Anxiety, Depression and Post Traumatic Stress Disorder after critical illness: a UK-wide prospective cohort study. Critical care (London, England). 2018;22(1):310.
45. Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185(12):1307-1315.
46. Del Rio C, Collins LF, Malani P. Long-term Health Consequences of COVID-19. Jama. 2020.
47. Carfì A, Bernabei R, Landi F. Persistent Symptoms in Patients After Acute COVID-19. Jama. 2020;324(6):603-605.
48. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nature Medicine. 2021;27(4):601-615.
49. The Lancet N. Long COVID: understanding the neurological effects. Lancet Neurol. 2021;20(4):247.
50. McLoughlin BC, Miles A, Webb TE, et al. Functional and cognitive outcomes after COVID-19 delirium. Eur Geriatr Med. 2020;11(5):857-862.
51. van den Borst B, Peters JB, Brink M, et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020.
52. Bellan M, Soddu D, Balbo PE, et al. Respiratory and Psychophysical Sequelae Among Patients With COVID-19 Four Months After Hospital Discharge. JAMA Netw Open. 2021;4(1):e2036142.
53. Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. Jama. 2010;304(16):1787-1794.
54. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629.
55. Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson LV. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):50-56.
56. Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF, Jr. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340-347.
57. Hughes CG, M, i A, et al. Association between endothelial dysfunction and acute brain dysfunction during critical illness. J Anesthesiology. 118(3):631-639.
58. Hui DS, Wong KT, Ko FW, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128(4):2247-2261.
59. Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15(3):543-550.
60. Zhang P, Li J, Liu H, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8:8.
61. Mak IW, Chu CM, Pan PC, Yiu MG, Chan VL. Long-term psychiatric morbidities among SARS survivors. Gen Hosp Psychiatry. 2009;31(4):318-326.
View all FLARE content
Learn more about research in the Division of Pulmonary and Critical Care Medicine