New Observational Reports on Anticoagulation and Immunomodulation
The FLARE Four
- There is intense interest in developing novel treatment strategies for COVID-19… and significant incentives for publishing reports of those strategies
- Notable papers from this week include observational data on therapeutic anticoagulation and immunomodulation in COVID-19
- The observational studies discussed in this FLARE have flaws in study design and analysis, which severely limit their utility in evaluating the proposed interventions
- Ultimately, only well-designed RCTs can determine the efficacy of new treatments for COVID-19
Many people are saying...several drugs show promise in COVID-19.
Subscribe to the latest updates from FLARE Advances in Motion
Introduction
During the deadly 2014 outbreak of Ebola Virus Disease (EVD), multiple experimental therapies were proposed (“2014-2016 Ebola Outbreak in West Africa | History | Ebola (Ebola Virus Disease) | CDC” 2020). The outbreak generated intense interest in providing such therapies outside the context of randomized controlled trials and, indeed, controversy erupted over whether RCTs were even ethical given the circumstances (Kalil 2020). Ultimately, only one RCT was conducted and no evidence-supported therapies were available at the time of the next outbreak of EVD in 2019.
The current SARS-CoV-2 pandemic has produced a similar sense of urgency among clinicians and researchers to develop, deploy, and report novel treatment strategies. Many centers are therefore using off-label therapies and reporting observational results in online pre-prints and journal articles. In tonight’s FLARE, we assess two recent observational papers in this mold - one on the use of therapeutic anticoagulation and another on the use of the IL-1 receptor antagonist anakinra. Due to the inherent limitations of observational data, neither of these reports should change prior probabilities for or against use of either of these agents. It is possible, however, that the attention devoted to them will result in practice changes. That being the case, one might fairly ask if we have reached the point in the pandemic where observational reports on therapeutic interventions are doing more harm than good.
Anticoagulation in COVID-19
The topic of anticoagulation in COVID-19 has been reviewed in prior FLAREs on March 30 and April 30. Briefly, biomarkers associated with thrombosis, such as d-dimer, are consistently elevated in COVID-19 (Spiezia et al. 2020) and multiple reports (Cui et al. 2020; Goyal et al. 2020; Klok et al. 2020) have suggested an elevated rate of thrombotic events (ranging from 7% to over 30%). In response, clinicians have advocated for protocols ranging from prophylactic anticoagulation (Tang et al. 2020) to full-dose therapeutic anticoagulation (Cattaneo et al. 2020), perhaps triggered by biomarkers such as d-dimer. To date, no randomized controlled trial data of specific anticoagulation strategies have been reported.
In a short letter published in the Journal of the American College of Cardiology (and the recipient of a great deal of popular press coverage), Paranjpe and colleagues describe the use of anticoagulation in a large observational cohort of 2773 patients treated within the Mount Sinai Health System in New York (Paranjpe et al. 2020). 28% of patients received some form of systemic anticoagulation during their hospital stay, with median time from admission to start of therapy of two days and median time on therapy of three days (interquartile range 2-7 days). Mortality was similar among patients who received therapeutic anticoagulation (AC) (22.5%) and those who did not (22.8%). Of note, patients who received therapeutic anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs 8.1%, p<0.001). Among mechanically ventilated patients, in-hospital mortality was remarkably high at 62.7% with a median survival of 9 days in the anticoagulation-free group. In contrast, 29.1% of ventilated patients receiving anticoagulation died during the follow up period. For comparison, mortality rate among ICU patients (not all of whom would have been intubated in all reports) with COVID-19 has been reported to range from 17% (Ziehr et al. 2020) to 67% (Yang et al. 2020). Reassuringly, the authors did not identify differential rates of major bleeding between anticoagulated (3%) and non-anticoagulated (2%) patients; this difference was not significant.
What Are We to Make of These Data?
Treatment decisions regarding anticoagulation should not be made based on these data. As the authors appropriately note, only a well-designed randomized control trial can offer definitive data on drug efficacy. The paper by Paranjpe and colleagues has methodological flaws which can result in misleading conclusions. We do not believe these data provide interpretable answers to the question of interest -- the therapeutic role of anticoagulation in patients with COVID-19.
Let’s start by reviewing the limitations as stated by the authors:
Confounding by indication: Patients who received anticoagulation received it because their caregivers chose to give it - presumably for a specific clinical indication. Additionally, patients who did not receive anticoagulation may have had a contraindication high bleeding risk. This is an issue inherent to all observational studies (in which exposure is not randomly allocated), though there are many methodological approaches to limiting such forms of confounding. However, the first step is for researchers and readers to understand the inherent differences between those patients exposed to the intervention of interest and those not exposed. If these groups are markedly different, one may not be able to draw any valid conclusions even with adjusted models and should consider a different approach to the research question (e.g. randomized trial, if feasible). Put simply, researchers must ask the question: “Why did clinicians choose to treat some patients with anticoagulation and not others?” Understanding the data, and the clinical context under which these were generated, is essential for good observational research. The authors do not provide any baseline patient characteristics, or subgroup characteristics among mechanically ventilated patients who did and did not receive anticoagulation. We have no way of knowing why some patients were treated and some were not. The paper does not provide data on institutional protocols, or anything else to help us understand any potential biases. Regardless, clinicians chose to give some patients anticoagulation because they thought they needed it, and chose to not give it to others -- i.e confounding by indication (Collet and Boivin 2000).
Unobserved confounding: It is notable that the reported mortality in the treatment arm tracks fairly closely with currently reported mortality in COVID-19 ICU patients, while mortality in the untreated arm is strikingly high. That observation does lend itself to the conclusion that there is something distinct about the two groups other than anticoagulation that confounds the results. Again, the authors do not provide readers with any ability to understand these between-group differences as no patient characteristics are provided.
Lack of metrics to classify disease severity: The high mortality of the untreated, mechanically ventilated patients is not addressed, nor is a detailed comparison made to the relatively low-mortality treated group. There is no description of the severity of respiratory failure. If this is to be a meaningful way to divide the patients, it is incumbent upon the authors to describe the very thing that identifies the patients, namely the presence of respiratory failure.
Other points not addressed by the authors include:
- The median duration of AC was 3 days (IQR 2-7 days); it seems highly biologically implausible that anticoagulation alone, provided for 3 days, would produce such a large treatment effect. We are not told, for instance, that there were a large number of deaths from thrombosis in the untreated arm. The lack of biological plausibility should heighten our suspicion that the effect is spurious (see below)
- We note that pulmonary hemorrhage is not included in the authors’ definition of major bleeding. This seems an important oversight
- Finally, and most importantly, immortal time bias is not addressed. This is a major threat to the validity of the study, to which we’ll devote the following section
Heparin and the Fall From Olympus
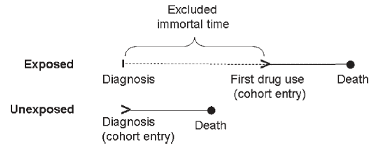
Figure 1
Illustration of immortal time bias (Suissa 2007).
Immortal time bias is a potential explanation for the implausibly large treatment effect that was, unfortunately, left unexamined by the authors (Suissa 2007). Immortal time bias (Figure 1) occurs in observational cohorts because the groups of interest are defined by their exposure (or lack of exposure) to the treatment at varied times. In contrast, clinical trials have a well-defined “time zero” when patients are enrolled and randomly assigned to a treatment.
In this report, patients are admitted to the hospital with COVID-19 and at some subsequent point they receive anticoagulation. Prior to having the order for anticoagulation written, no patient in the treatment group can die - because if they did die prior to that point, that death would have been assigned to the “no anticoagulation” group rather than the “anticoagulation” group. Thus the treatment group benefits from getting all the patients who survived long enough to develop an indication for anticoagulation and receive anticoagulation. All the “immortal time” accrued prior to treatment serves to benefit the treatment group in terms of the apparent mortality or survival.
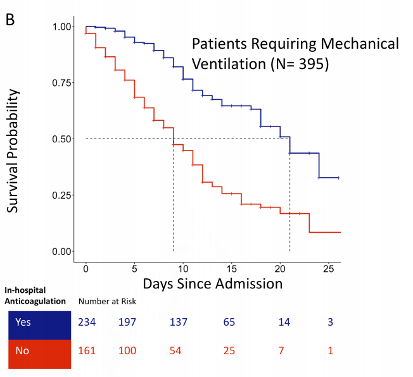
Figure 2
Kaplan-Meier survival curve for patients requiring mechanical ventilation (Paranjpe et al. 2020).
Given that we are not provided details on the patients in each group, we can look to the Kaplan-Meier curve (Figure 2) to generate some hypotheses about bias. It is striking that within 3-4 days of admission, about 20% of the patients in the no anticoagulation group have died. Yet the anticoagulation group remains, nearly universally, alive through day 5 before deaths begin to occur. Why? Because a patient who survived 5 days (or 3 days, or 7 days, etc), then on hospital day 5 received anticoagulation, is assigned to the “anticoagulation” group. In other words, anticoagulation “gets the credit” for those 5 days of survival despite having nothing to do with it. Conversely, the “no anticoagulation” arm is assigned all of those early deaths. We cannot emphasize this enough -- studies which seek to determine treatment effects need a clearly defined time zero to avoid this fatal flaw of immortal time bias (Hernán et al. 2016).
The authors additionally use “days of anticoagulation” as an exposure in their model. This is problematic, and could lead to conclusions similar to associating “survival” with “number of daily progress notes written for the patient.” You must be alive to receive the exposure, anticoagulation. If you live longer, you receive more days of anticoagulation regardless of why you lived longer. Therefore this is a self-fulfilling prophecy. Patients who continue to be alive in the cohort receive more anticoagulation, and therefore the model associates anticoagulation with lower daily hazard ratio for death.
Immortal time bias can lead to large and deeply misleading conclusions. In the case of the Paranjpe study, it is quite likely that the primary effect of anticoagulation was to cast the patients in that arm from Mount Olympus, cruelly terminating their immortality. We await randomized controlled trial data to determine the utility of anticoagulation in COVID-19. Fortunately, ClinicalTrials.gov currently lists twelve active studies designed to answer this specific question. Given that we will shortly have high quality data on the utility of various anticoagulation strategies, it seems best to disregard signals from observational trials such as this.
Immortality, Schrodinger's Ventilator (Again!) and Immunomodulation in COVID-19
A second observational drug report (Cavalli et al. 2020) out this week involves immune modulation with the IL-1 receptor antagonist anakinra. The putative rationale for this therapy was reviewed in prior FLARE (March 25). Briefly, it is well established that patients with COVID-19 have elevated markers of systemic inflammation and that the levels of these markers are correlated with outcome (Wu et al. 2020). Less established is the significance or treatment implications of this association. It has been argued (Mehta et al. 2020) that the spectrum of abnormal laboratory values in COVID-19 is similar to that observed in so-called secondary HLH, macrophage activation syndrome (MAS) or cytokine release syndrome as seen, for example, as a CAR-T cell therapy complication. Although this analogy is frequently made in sepsis from various causes and now in COVID-19, it is not well founded. As outlined in our prior FLARE, the disordered T-cell immunity which characterizes sHLH in the setting of CAR T-cell infusion (a therapy predicated upon the deliberate induction of T-cell mediated immunity) is highly unlikely to be a significant component of COVID-19 or other forms of sepsis, superficial lab similarities aside.
Whatever one’s prior beliefs on the utility of immunomodulation in COVID-19, it is worth taking note of the randomized trial data which are available on the use of anakinra in sepsis. Clearly these trials did not involve COVID-19 patients, but the septic shock was similarly hypothesized to be from a disordered immune response. As we outlined both on March 25 and April 4, Fisher and colleagues (Fisher 1994) conducted a randomized controlled trial of intravenous anakinra in 893 septic patients and found no difference in 28-day all-cause mortality. A second randomized controlled trial of intravenous anakinra was conducted by Opal and colleagues (Opal et al. 1997) among patients who were considered to be at higher risk of death. This trial was negative as well.
Much of the current interest in the use of anakinra in COVID-19 stems from a post hoc subgroup analysis of prior trials in which features of hepatobiliary dysfunction and/or DIC were associated with reduced mortality (Shakoory et al. 2016). The subgroup was not, notably, defined by the spectrum of elevated cytokines actually observed in COVID-19 (particularly CRP and ferritin).
Based on these rather equivocal results, Cavalli and colleagues sought to determine the effect of anakinra on patients with COVID-19 and performed a single center, observational study in Italy carried out in two stages. They initially studied seven patients with COVID-19 complicated by moderate to severe ARDS (per ARDS Definition Task Force et al. 2012) and hyperinflammation (serum C-reactive protein ≥ 100 mg/L, ferritin ≥ 900 ng/mL, or both) who were being managed outside of the ICU with non-invasive positive pressure ventilation. All patients were also receiving hydroxychloroquine and lopinavir/ritonavir. These seven patients were given 100mg of subcutaneous anakinra twice daily and compared to a contemporaneous group of 16 patients who did not receive anakinra. After one week, they found no difference between the groups in inflammatory markers or degree of respiratory failure. In response, they treated an additional 29 patients with an extremely high dose of intravenous anakinra (5 mg/kg twice daily) and compared them to the prior, untreated group. The authors report that none of these patients were from the low dose anakinra group but we are not told how they were selected or when they were admitted to the hospital. They report 90% survival in the high-dose IV anakinra group at 21 days and 56% in the standard treatment group (associated with a p-value of 0.009). They further report a greater degree of improvement in respiratory failure (as measured by a standard, 7-pt scale) in the treatment group (72% of patients improved in the anakinra group and 50% in the untreated group). Bacteremia and hepatic injury led to discontinuation of anakinra in some patients, but these complications did not occur at a higher rate than in the control group.
It is very difficult to conclude anything meaningful from these data. The authors acknowledge some important limitations to their study: its retrospective nature, small sample size, and limited follow-up. In addition, no information is provided about the date of admission, the duration of hospitalization prior to treatment (or not) with anakinra, or length of hospitalization for any of the three groups. It is therefore not possible to rule out that this paper suffers from the same sort of immortal time bias as the report Paranjpe and colleagues. Moreover, the reported mortality numbers suffer from a familiar problem - a disproportionate use of Schroedinger’s ventilator in the treatment arm. If we look at table 3:
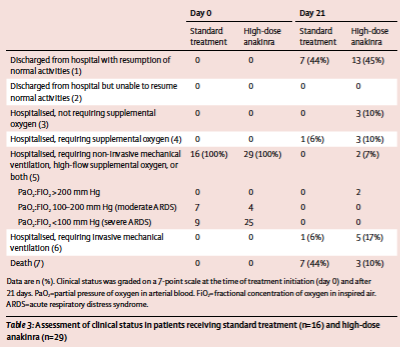
Table 1
Table from Cavalli et al. 2020.
We see that at the end of the 21-day follow-up period, 5 patients (17%) in the treatment group were still hospitalized and were receiving invasive mechanical ventilation (and 2 were still receiving non-invasive positive pressure ventilation), while just 1 in the control arm was. These patients are sensibly not counted towards the mortality in the treatment arm (as they are not dead) but they have yet to reach a definitive outcome - they are neither alive nor dead. If we apply a composite endpoint of mechanical ventilation or death, we find a more pedestrian difference between groups of 50% (8 of 16) death or mechanical ventilation in the un-treated arm and 28% (8 of 29) in the treated arm - a difference which is no longer statistically significant (p = 0.15). In other words - it is fair to ask if the difference between the two arms is driven in part by differential use/length of ventilation. Causes of death were similar between the two arms (pulmonary embolism, respiratory failure, multiorgan failure) and the authors acknowledged the severe resource constraints around mechanical ventilation in their hospital at the time. Given that these patients all had moderate-to-severe ARDS and were being treated with NIPPV, it is not surprising that a sizable percentage of them ultimately required invasive mechanical ventilation (see May 7 FLARE). However, we are not given enough clinical details to conclude if there were temporal changes in the use of mechanical ventilation that might, in part, explain the difference between the groups.
In short, this is an observational trial for a drug which has failed in multiple, large randomized controlled trials. Though there might exist a subset of patients who benefit from anti-IL-1 receptor therapy, performing a retrospective cohort study of a small group of patients is extremely unlikely to identify them. We are in vigorous agreement with the authors’ assertion that these results are preliminary and require much more rigorous study. We share their eagerness for randomized, controlled data.
Conclusions
Given the devastating impact of the COVID-19 pandemic, there is an understandable desire to try new therapies, many on an off-label or “compassionate use” basis. In addition to the unknown dangers associated with novel therapies, we should consider the danger posed by the publication of easy to misinterpret data. Unlike the outbreak of EVD, we have very effective, standard protocols for the treatment of COVID-19 ARDS - protocols which have been reported to be associated with a mortality similar to those reported in the treatment arms of tonight's papers (Ziehr et al. 2020; Goyal et al. 2020; Grasselli et al. 2020). Given this, we should insist on a high standard of evidence prior to incorporating new strategies, and we should be alert to the risks of overlooking flaws in observational data whose conclusions align with our priors. Extraordinary efforts have been made to facilitate the rapid design and conduct of randomized trials in COVID-19 and the high volume of patients will allow RCT data to answer many of the questions we are currently struggling with. Indeed, it is fair to ask what has gone wrong when the results of a phase III, randomized control trial of a plausible therapy are available only as a press release, while the results of difficult to interpret, observational studies are rapidly published and widely read. The incentives that result in this perverse situation are many. However, given the intense interest in any news around COVID-19, we must hold ourselves to the highest scientific standards, report valid data, and allow proper ongoing clinical trials to determine drug treatment effects. Now is the time to develop rigorous data so that we are prepared with answers to important questions prior to the next phase of the pandemic.
References:
- “2014-2016 Ebola Outbreak in West Africa | History | Ebola (Ebola Virus Disease) | CDC.” 2020. March 17, 2020. https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/index.html.
- ARDS Definition Task Force, V. Marco Ranieri, Gordon D. Rubenfeld, B. Taylor Thompson, Niall D. Ferguson, Ellen Caldwell, Eddy Fan, Luigi Camporota, and Arthur S. Slutsky. 2012. “Acute Respiratory Distress Syndrome: The Berlin Definition.” JAMA: The Journal of the American Medical Association 307 (23): 2526–33.
- Cattaneo, Marco, Elena M. Bertinato, Simone Birocchi, Carolina Brizio, Daniele Malavolta, Marco Manzoni, Gesualdo Muscarella, and Michela Orlandi. 2020. “Pulmonary Embolism or Pulmonary Thrombosis in COVID-19? Is the Recommendation to Use High-Dose Heparin for Thromboprophylaxis Justified?” Thrombosis and Haemostasis, April. https://doi.org/10.1055/s-0040-1712097.
- Cavalli, Giulio, Giacomo De Luca, Corrado Campochiaro, Emanuel Della-Torre, Marco Ripa, Diana Canetti, Chiara Oltolini, et al. 2020. “Interleukin-1 Blockade with High-Dose Anakinra in Patients with COVID-19, Acute Respiratory Distress Syndrome, and Hyperinflammation: A Retrospective Cohort Study.” The Lancet Rheumatology, May. https://doi.org/10.1016/S2665-9913(20)30127-2.
- Collet, Jean-Paul, and Jean-François Boivin. 2000. “Bias and Confounding in Pharmacoepidemiology.” In Pharmacoepidemiology, edited by Brian L. Strom, 765–84. Chichester, UK: John Wiley & Sons, Ltd.
- Cui, Songping, Shuo Chen, Xiunan Li, Shi Liu, and Feng Wang. 2020. “Prevalence of Venous Thromboembolism in Patients with Severe Novel Coronavirus Pneumonia.” Journal of Thrombosis and Haemostasis: JTH, April. https://doi.org/10.1111/jth.14830.
- Fisher, C. J. 1994. “Recombinant Human Interleukin 1 Receptor Antagonist in the Treatment of Patients with Sepsis Syndrome. Results from a Randomized, Double-Blind, Placebo-Controlled Trial. Phase III rhIL-1ra Sepsis Syndrome Study Group.” JAMA: The Journal of the American Medical Association. https://doi.org/10.1001/jama.271.23.1836.
- Goyal, Parag, Justin J. Choi, Laura C. Pinheiro, Edward J. Schenck, Ruijun Chen, Assem Jabri, Michael J. Satlin, et al. 2020. “Clinical Characteristics of Covid-19 in New York City.” The New England Journal of Medicine, April. https://doi.org/10.1056/NEJMc2010419.
- Grasselli, Giacomo, Alberto Zangrillo, Alberto Zanella, Massimo Antonelli, Luca Cabrini, Antonio Castelli, Danilo Cereda, et al. 2020. “Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy.” JAMA: The Journal of the American Medical Association, April. https://doi.org/10.1001/jama.2020.5394.
- Kalil, Andre C. 2020. “Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics.” JAMA: The Journal of the American Medical Association, March. https://doi.org/10.1001/jama.2020.4742.
- Klok, F. A., M J H, N. J. M. van der Meer, M. S. Arbous, D. Gommers, K. M. Kant, F. H. J. Kaptein, et al. 2020. “Confirmation of the High Cumulative Incidence of Thrombotic Complications in Critically Ill ICU Patients with COVID-19: An Updated Analysis.” Thrombosis Research. https://doi.org/10.1016/j.thromres.2020.04.041.
- Mehta, Puja, Daniel F. McAuley, Michael Brown, Emilie Sanchez, Rachel S. Tattersall, Jessica J. Manson, and HLH Across Speciality Collaboration, UK. 2020. “COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression.” The Lancet 395 (10229): 1033–34.
- Opal, Steven M., Charles J. Fisher, Jean-Francois A. Dhainaut, Jean-Louis Vincent, Rainer Brase, Stephen F. Lowry, Jerald C. Sadoff, et al. 1997. “Confirmatory Interleukin-1 Receptor Antagonist Trial in Severe Sepsis: A Phase III, Randomized, Doubleblind, Placebo-Controlled, Multicenter Trial.” Critical Care Medicine 25 (7): 1115.
- Paranjpe, Ishan, Valentin Fuster, Anuradha Lala, Adam Russak, Benjamin S. Glicksberg, Matthew A. Levin, Alexander W. Charney, et al. 2020. “Association of Treatment Dose Anticoagulation with In-Hospital Survival Among Hospitalized Patients with COVID-19.” Journal of the American College of Cardiology, May. https://doi.org/10.1016/j.jacc.2020.05.001.
- Shakoory, Bita, Joseph A. Carcillo, W. Winn Chatham, Richard L. Amdur, Huaqing Zhao, Charles A. Dinarello, Randall Q. Cron, and Steven M. Opal. 2016. “Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial.” Critical Care Medicine 44 (2): 275–81.
- Spiezia, Luca, Annalisa Boscolo, Francesco Poletto, Lorenzo Cerruti, Ivo Tiberio, Elena Campello, Paolo Navalesi, and Paolo Simioni. 2020. “COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure.” Thrombosis and Haemostasis, April. https://doi.org/10.1055/s-0040-1710018.
- Suissa, Samy. 2007. “Immortal Time Bias in Observational Studies of Drug Effects.” Pharmacoepidemiology and Drug Safety. https://doi.org/10.1002/pds.1357.
- Tang, Ning, Huan Bai, Xing Chen, Jiale Gong, Dengju Li, and Ziyong Sun. 2020. “Anticoagulant Treatment Is Associated with Decreased Mortality in Severe Coronavirus Disease 2019 Patients with Coagulopathy.” Journal of Thrombosis and Haemostasis: JTH 18 (5): 1094–99.
- Wu, Chaomin, Xiaoyan Chen, Yanping Cai, Jia ’an Xia, Xing Zhou, Sha Xu, Hanping Huang, et al. 2020. “Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China.” JAMA Internal Medicine, March. https://doi.org/10.1001/jamainternmed.2020.0994.
- Yang, Xiaobo, Yuan Yu, Jiqian Xu, Huaqing Shu, Jia ’an Xia, Hong Liu, Yongran Wu, et al. 2020. “Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study.” The Lancet. Respiratory Medicine 8 (5): 475–81.
- Ziehr, David R., Jehan Alladina, Camille R. Petri, Jason H. Maley, Ari Moskowitz, Benjamin D. Medoff, Kathryn A. Hibbert, B. Taylor Thompson, and C. Corey Hardin. 2020. “Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study.” American Journal of Respiratory and Critical Care Medicine, April. https://doi.org/10.1164/rccm.202004-1163LE.
View all COVID-19 updates
Learn more about research in the Division of Pulmonary and Critical Care Medicine