Poster: A Clinical and Biomarker Scoring System to Predict the Presence of Obstructive PAD
In This Article
- Peripheral artery disease (PAD) is a global health problem with 202 million people living with the diagnosis
- As symptoms of PAD are protean, its diagnosis is challenging until it’s in the advanced stages
- In a prospective cohort of 355 patients referred for diagnostic peripheral angiography and/or coronary angiography enrolled, predictors of =50% stenosis in at least one peripheral vessel were identified from over 50 clinical variables and 109 biomarkers
- A score derived from the final model was built with the entire population, and evaluated within the same population to predict obstructive PAD
- Clinically, use of a tool such as this could act as a gatekeeper prior to imaging or invasive testing, thus reducing cost and exposures to intravenous contrast and/or ionizing radiation
Subscribe to the latest updates from Cardiovascular Advances in Motion
Peripheral artery disease (PAD) is an increasing global health problem with 202 million people living with the diagnosis. As symptoms of PAD are protean, its diagnosis is challenging until it’s in the advanced stages. Because of this, PAD is often underdiagnosed and undertreated, and most patients are not receiving optimal management.
Non-invasive tools to predict the presence and severity of PAD have limitations including inaccuracy, cost and need to utilize intravenous contrast and/or ionizing radiation. A need exists for alternative means for evaluating PAD. One option might be use of biomarkers to identify presence and prognosticate course of the diagnosis.
Methods
In a prospective cohort of 355 patients referred for diagnostic peripheral angiography and/or coronary angiography enrolled in the Catheter Sampled Blood Archive in Cardiovascular Diseases Study, predictors of ≥50% stenosis in at least one peripheral vessel were identified from over fifty clinical variables and 109 biomarkers.
15 mL of blood was obtained immediately before angiography from each patient and processed immediately. Biomarkers were measured.
Predictive models were generated using LASSO with logistic regression. A score derived from the final model was built with the entire population, and evaluated within the same population to predict obstructive PAD. Furthermore, Kaplan-Meier survival curves were used to assess time to revascularization as a function of the clinical/biomarker score.
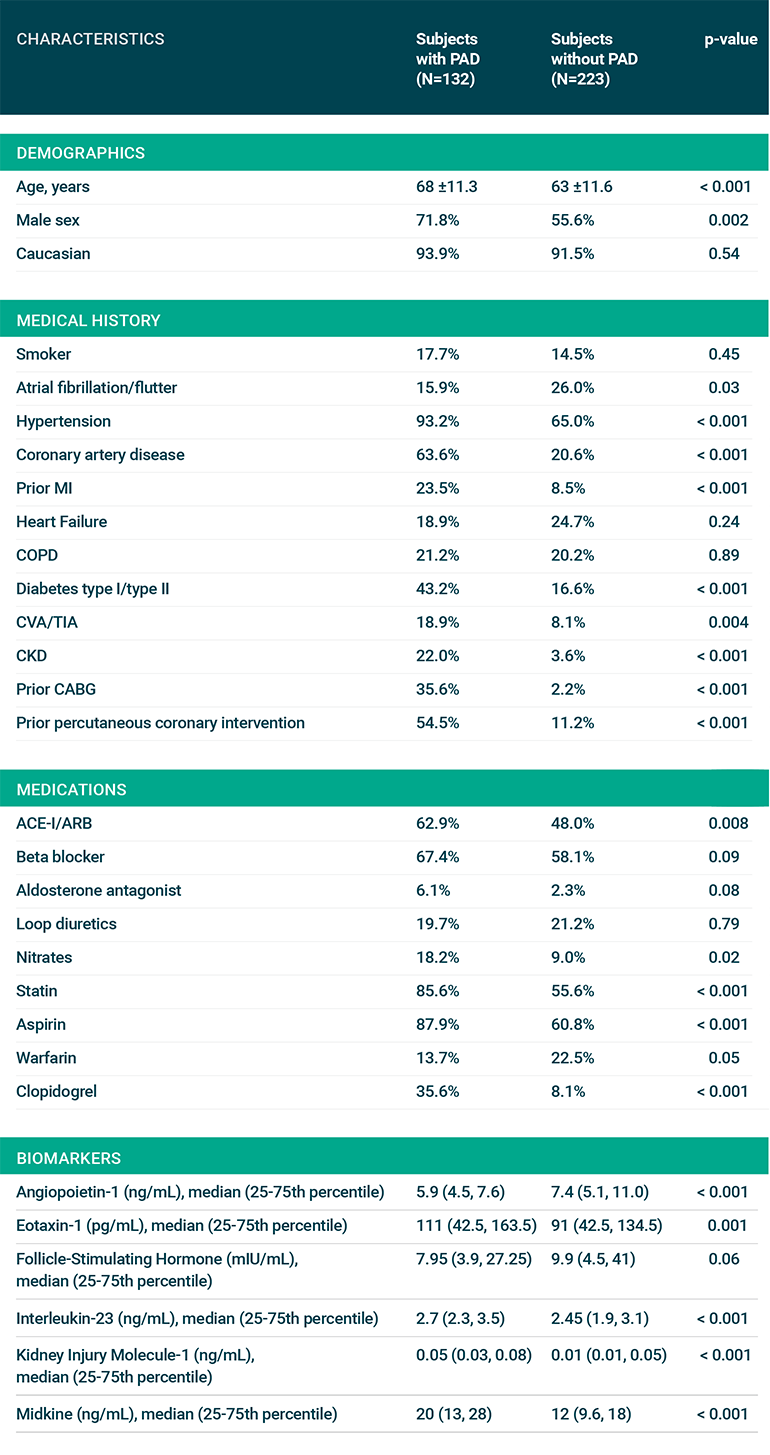
Fig 1: Baseline Characteristics
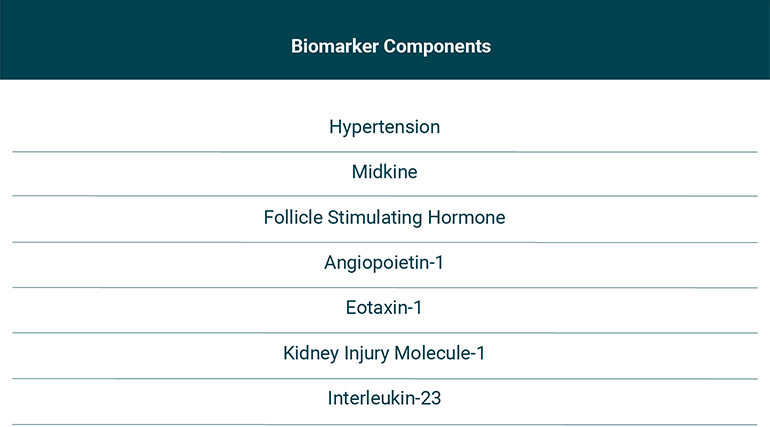
Fig 2: Components of the clinical/biomarker score
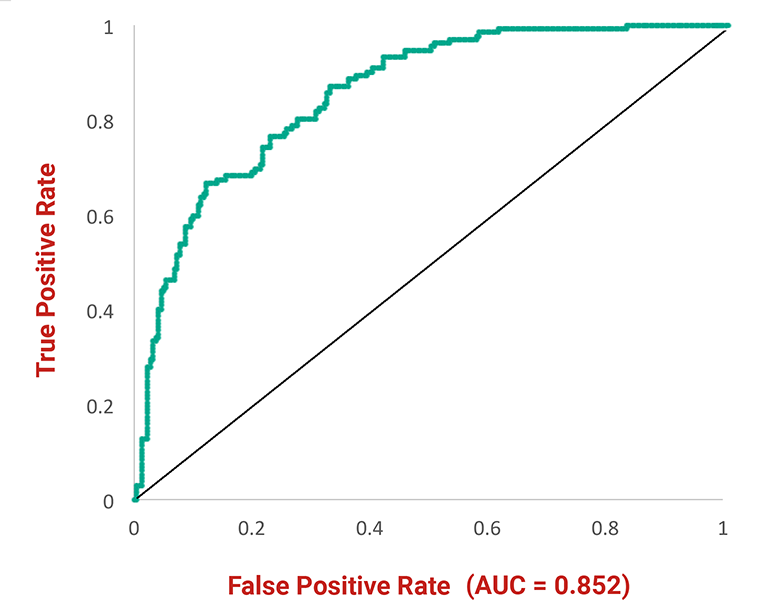
Fig 3: Receiver operating characteristic curve
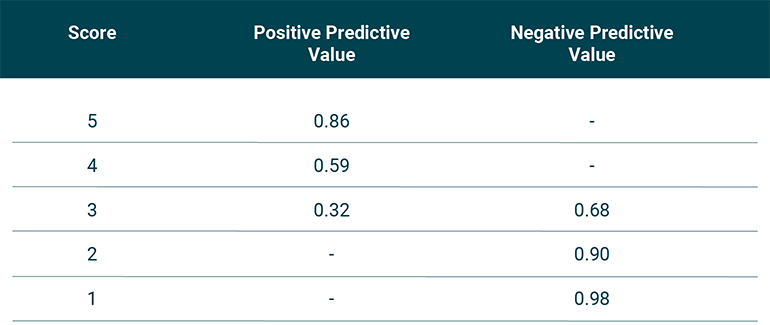
Fig 4: Predictive values of each score
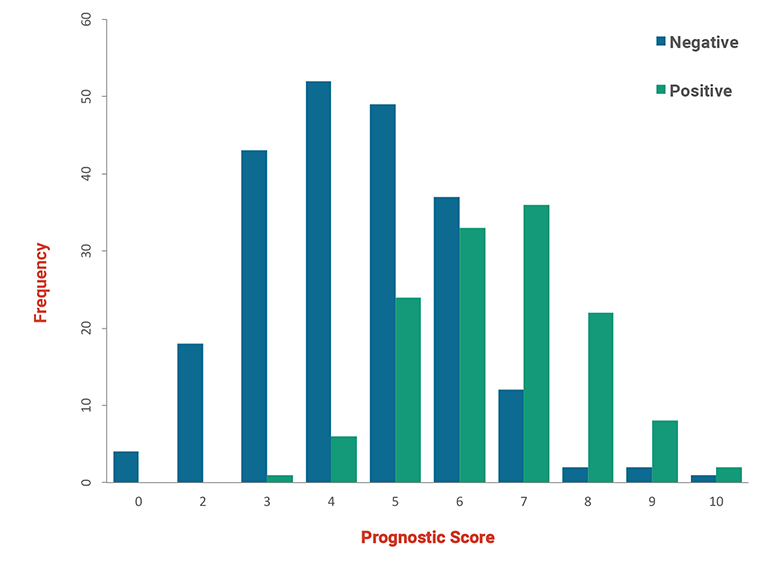
Fig 5: Histogram
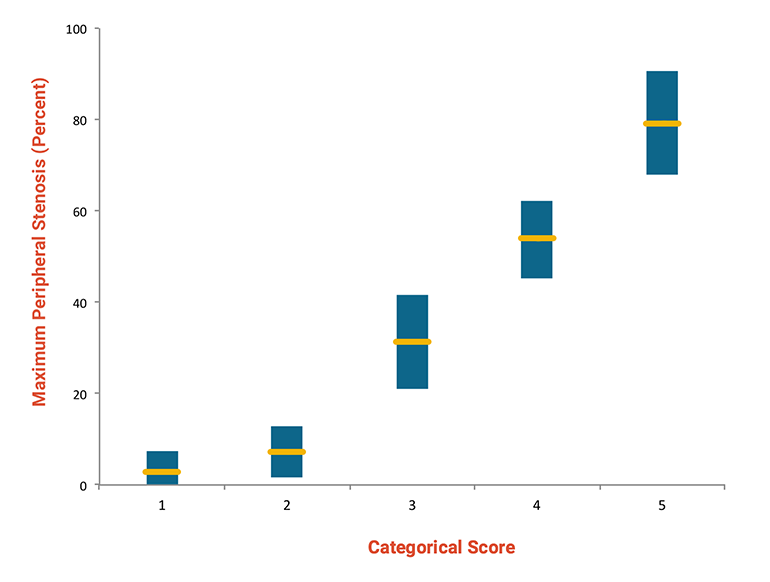
Fig 6: Correlation of score with stenosis
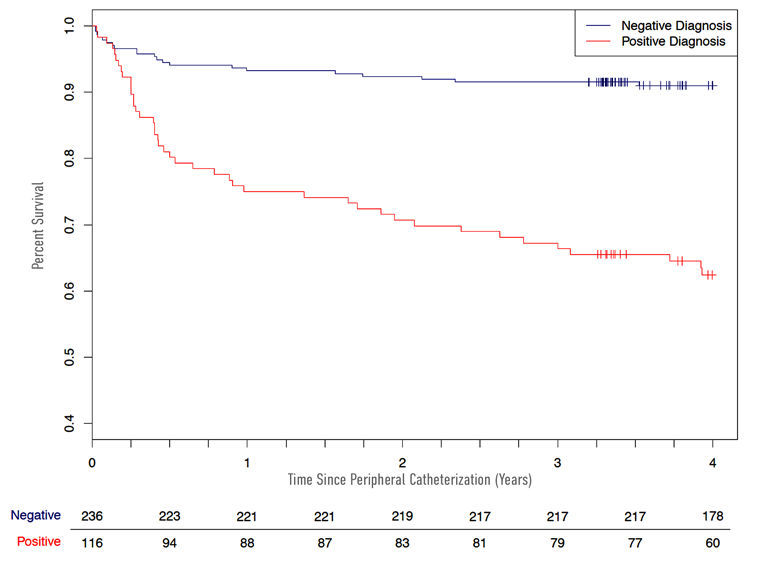
Fig &: Time to revascularization
Limitations
Biomarkers were measured at a single point in time, which may not reflect levels at future time periods.
Our results need further validation in larger cohorts.
Conclusion
In a prospective cohort study, we describe a novel method to predict angiographically significant PAD, also lending potential prognostic information regarding need for revascularization.
The biomarkers in this model all have plausible biologic links to atherosclerosis.
Clinically, use of a tool such as this could act as a gatekeeper prior to imaging or invasive testing, thus reducing cost and exposures to intravenous contrast and/or ionizing radiation.
The score may also be used to evaluate at-risk patients for risk of vascular complications; as such a role in clinical trials to enrich for PAD-related events or to identify patients at risk for adverse effects of drug therapies is plausible.
Authors
Cian P. McCarthy MB, BCh, BAOa, Nasrien E. Ibrahim, MDb, Roland R.J. van Kimmenade MD, PhDc, Hanna K. Gaggin, MD, MPHb,d, Mandy L. Simon, DNP, FNP-BCb, Parul Gandhi, MDe, Noreen Kelly, MDf, Shweta R. Motiwala, MDg, Jamie Harisiades, BAb, Renata Mukai, BAb, Craig A. Magaret, MSh, Grady Barnes, PhDh, Rhonda F. Rhyne, B.Pharm, MBAh, Joseph M. Garasic, MDb and James L. Januzzi Jr MDb,d.
aDepartment of Medicine, Massachusetts General Hospital, Boston, Massachusetts, United States of America, bDivision of Cardiology, Massachusetts General Hospital, Boston, Massachusetts, United States of America, cDivision of Cardiology, Maastricht University Medical Centre, Maastricht, the Netherlands, dBaim Institute for Clinical Research, Cardiometabolic Trials, Boston, Massachusetts, United States of America, eDivision of Cardiology, VA Connecticut Healthcare System and Yale University, New Haven, Connecticut, United States of America, fDivision of Cardiology, Brigham and Women's Hospital, Boston, Massachusetts, United States of America, gDivision of Cardiology, Beth Israel Deaconess Medical Center, Boston, Massachusetts, United States of America, hPrevencio, Inc, Kirkland, Washington, United States of America.
Funding Disclosures: Dr Ibrahim is supported by the Dennis and Marilyn Barry fellowship in cardiology research. Dr. Januzzi is supported in part by the Hutter Family Professorship in Cardiology. Dr. Gaggin is supported in part by the Ruth and James Clark Fund for Cardiac Research Innovation. This work was supported by a grant from Prevencio, Inc.
Disclosures: Dr. Januzzi has received grant support from Roche Diagnostics, Siemens, Cleveland Heart Labs and Prevencio, consulting income from Roche Diagnostics, Critical Diagnostics, Phillips, and Novartis, and participates in clinical endpoint committees/data safety monitoring boards for Abbvie, Bayer, Pfizer, Novartis, Amgen, Janssen, and Boehringer Ingelheim. Mr. Magaret is a consultant to Prevencio, Inc. Dr. Gaggin has received grant support from Roche and Portola; consulting income from Roche Diagnostics, American Regent, Amgen, Boston Heart Diagnostics and Critical Diagnostics; research payments for clinical endpoint committees for EchoSense. Dr. Garasic has received consulting income from Siemens, Applied Clinical Intelligence, Bayer and Merck, Boehringer Ingelheim and AbbVie. Ms. Rhyne and Dr. Barnes are employees of Prevencio, Inc. The other authors have nothing to disclose.
Visit the Corrigan Minehan Heart Center
Refer a patient to the Corrigan Minehan Heart Center