Stem Cells and COVID-19
The FLARE Four
- Based on small case series, MSCs have been proposed as an immunomodulatory therapy for COVID-19
- The literature on MSCs suffers from a lack of consensus on the definition of MSC and vague descriptions of their mechanism of action
- Phase 1 and Phase 2a clinical trials of MSC therapy in ARDS demonstrated MSC safety but did not demonstrate efficacy
- Even though MSC therapy has not shown benefit for any human disease in a rigorous setting, clinical trials of MSCs are enrolling COVID-19 patients throughout the world
Many people are asking...can we use mesenchymal stem cells (MSCs) to cure COVID-19?
Subscribe to the latest updates from FLARE Advances in Motion
Introduction
Bone-marrow derived stem cells have been proposed as potential therapies for a wide variety of ailments, from rotator cuff injury to Alzheimer’s disease. In the context of the COVID-19 pandemic, mesenchymal stem cells (MSCs) have been proposed as a therapy for COVID-19 associated respiratory failure. In this FLARE, we describe the context behind these proposals.
Stem Cell Definitions (NIH Stem Cell Primer):
Stem cells have two key properties: they renew themselves through cell division, and they develop into different specialized cell types. Embryonic stem cells (ESCs) comprise the most well-established pool of stem cells, since each ESC can give rise to all of the cell types of an entire organism. In contrast, stem cells in adult tissues, such as lung, gut, and skin, do not have the potential to contribute to distinct tissue types, but rather they locally replenish cells of the same tissue type. Hematopoietic stem cells (HSCs) give rise to all of the specialized cell types of blood and are one of the few examples of well-established stem cell therapies. Importantly, these cells do not give rise to non-blood cell types.
The mesenchyme is a tissue derived from the embryonic mesoderm that eventually gives rise to the skeleton and connective tissues, including the blood system. Mesenchymal stromal cells are cells found in connective tissues that, like adult stem cells, have a limited capacity for differentiating into related mesoderm-derived tissues, such as cartilage, bone, and fat.
Unlike HSCs and ESCs, these mesenchymal stromal cells (MSCs) are not well defined by their function in vivo and have a confusing history surrounding their nomenclature. Early studies suggested that mesenchymal stromal cells could replace damaged tissue elsewhere in the body, and thus became known as mesenchymal stem cells. The name of this heterogeneous population of cells has varied over the years, spanning Marrow Stromal Cells, Multipotent Stromal Cells, Mesodermal Stem Cells, Mesenchymal Stromal Cell, and most recently, Medicinal Signaling Cells (Caplan, 2017).
Are MSCs “Regenerative,” “Immunomodulatory,” Both, or Neither?
The regenerative potential of bone marrow-derived stem cell therapy provided the initial excitement for this field. Early animal models and even small clinical studies suggested transdifferentiation of exogenous stem cells to cells of injured organs, including the lung (Kotton et al., 2001). However, more rigorous studies, sometimes from the same groups, almost uniformly refuted the notion that exogenous stem cells would simply replace the cells in the injured organ (Kotton et al., 2005). In fact, while negative results are always difficult to prove, MSCs have been consistently shown not to contribute to tissue regeneration outside of the bone marrow.
Over the last 10-15 years there appears to have been a “rebranding” of MSC therapy with less emphasis on their potential regenerative capacity and much more emphasis on their “immunomodulatory” potential. MSCs have been alleged to secrete trophic (“nurturing”), immunomodulatory, anti-scarring and chemoattractant factors (Caplan and Correa, 2011; Caplan and Dennis, 2006; da Silva Meirelles et al., 2009). Some have argued that “immunomodulation” properties of MSCs derive from secretion of extracellular vesicles that contain a variety of “immunomodulation factors” (Abraham and Krasnodembskaya, 2020; Witwer et al., 2019).
Thus, evaluating the literature on MSCs is uniquely difficult because of shifting definitions of MSCs and the challenge of standardizing protocols for cell isolation and preparation. In turn, these challenges provide a crutch for nearly every negative result. Regardless of cell preparation, there is consensus that, other than HSCs in the bone marrow, MSCs do not directly repair any injured organs through direct differentiation. Whether MSCs remain alive after infusion, whether they communicate with host cells, whether they secrete any therapeutic molecules and for how long remains largely unknown.
MSCs and ARDS: The START Study
Even prior to COVID-19, MSC therapy was being investigated in ARDS. Pre-clinical data were derived from various animal models of critical illness including a study of hemorrhagic shock-induced ARDS in rats, where MSCs were found to decrease lung inflammation and increase endothelial integrity (Matthay et al., 2017; Pati et al., 2011). After a Phase I dose-escalation trial (Wilson et al., 2015), the START investigators enrolled patients with moderate to severe ARDS into a meticulously-designed, prospective, double-blind, multicenter, randomized Phase 2a trial. The primary endpoint was safety, with intention to treat analysis. MSC source was allogeneic bone marrow-derived stromal cells from a healthy donor. Perhaps a sign of the times, MSCs are described as “mesenchymal stem cells” in the Phase 1 trial (Wilson et al., 2015) and “mesenchymal stromal cells” in the Phase 2a trial (Matthay et al., 2019).
The primary result of the Phase 2a START study was that a single injection of MSCs during ARDS is safe. A more nuanced review of the START study results may still raise safety concerns about MSCs, given a non-significant increase in mortality in the MSC group, although patients in the MSC cohort were also somewhat sicker at baseline. Some biomarker levels changed in the treatment group after the MSC infusion, but the significance of these secondary, surrogate endpoints is completely unknown.
There are two important lessons from this 2019 study. First, this trial provided a clear framework for conducting a rigorous MSC efficacy trial. Second, as a Phase 2 trial, START did not demonstrate efficacy. A phase 2 study does not exclude the possibility of finding efficacy in an appropriately powered trial, but it is worth noting that no dramatic effect was seen. MSCs are certainly not a panacea for patients with moderate to severe ARDS.
Why Is There Special Interest in the Use of Mscs in COVID-19 Therapy?
To explain poor outcomes in severe COVID-19, some have invoked a dysregulated immune response and “cytokine storm.” This has led to many proposals for “immunomodulation” as a therapeutic strategy in critically-ill COVID-19 patients (March 25 FLARE). As such, MSCs are being explored as potential COVID-19 therapy (Figure). Furthermore, proponents of MSCs argue that intravenously injected MSCs “home” to the injured lung. Of note, even if MSCs are found in the lung after injection, whether this results from “homing” to the injury site or simple embolization of a pulmonary blood vessel by a clump of live or dead cells, is unknown.
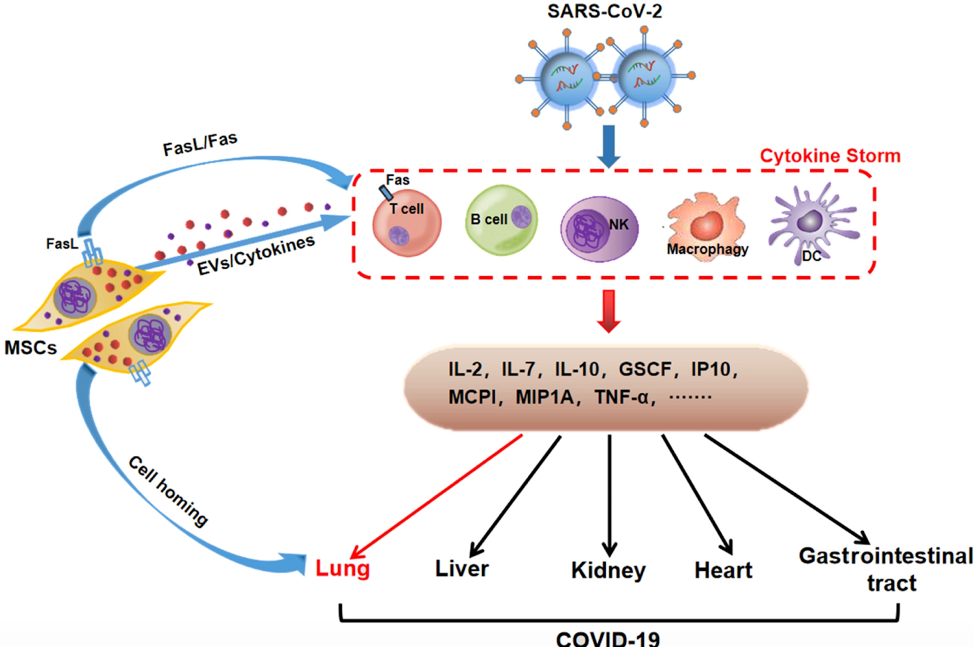
Figure 1
Proposed effects of MSCs on immune response in SARS-CoV-2 infection (Ji et al., 2020).
Currently, there is one published case series of seven COVID-19 patients (Leng et al., 2020) treated with MSCs, only one of whom was critically ill. In the case series, seven patients received an infusion of MSCs during their hospitalization for COVID-19. This study is neither randomized nor blinded and has many other major methodological problems. However, these shortcomings did not prevent the authors - and many reviews - from concluding that: “the intravenous transplantation of MSCs was safe and effective for treatment in patients with COVID-19 pneumonia”! Subsequently, many clinical trials have been, in part, inspired by this case series. Currently there are multiple trials of MSCs for COVID-19 listed on clinicaltrials.gov.
It should come as no surprise that any anecdotal examples of “stem cell therapy for COVID-19” are also quickly picked up by the media, with absurd but predictable headlines, such as “COVID-19 treatment with 100% survival rate” from placenta-derived stem cell treatment of four patients for “compassionate use,” apparently outside of a clinical trial.
Conclusions
Before COVID-19, clinical trials of MSCs in ARDS demonstrated safety and established a clear framework for conducting a rigorous MSC efficacy trial. There may be temptation to treat COVID-19 patients with MSCs through a “compassionate use protocol” before any results on the efficacy of MSCs in COVID-19 become available. This would be unwise. There is no signal in the available research on MSCs in ARDS (or COVID-19) supporting a presumption of efficacy. Widespread adoption of poorly premised approaches has plagued the recent evaluation of other COVID-19 therapies such as hydroxychloroquine and remdesivir. We can strive to do better. Given the large volume of COVID-19 patients, investigators could answer the question of MSC efficacy within several months! Indeed, clinical trials aim to use MSC either to suppress or to boost the immune system during a COVID-19 infection. Pending the results of these trials, there is no current indication for use of MSCs in COVID-19.
References:
- Abraham, A., and Krasnodembskaya, A. (2020). Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl. Med. 9, 28–38.
- Caplan, A.I. (2017). Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 6, 1445–1451.
- Caplan, A.I., and Correa, D. (2011). The MSC: an injury drugstore. Cell Stem Cell 9, 11–15.
- Caplan, A.I., and Dennis, J.E. (2006). Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 98, 1076–1084.
- Ji, F., Li, L., Li, Z., Jin, Y., and Liu, W. (2020). Mesenchymal stem cells as a potential treatment for critically ill patients with coronavirus disease 2019. Stem Cells Transl. Med.
- Kotton, D.N., Ma, B.Y., Cardoso, W.V., Sanderson, E.A., Summer, R.S., Williams, M.C., and Fine, A. (2001). Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 128, 5181–5188.
- Kotton, D.N., Fabian, A.J., and Mulligan, R.C. (2005). Failure of bone marrow to reconstitute lung epithelium. Am. J. Respir. Cell Mol. Biol. 33, 328–334.
- Leng, Z., Zhu, R., Hou, W., Feng, Y., Yang, Y., Han, Q., Shan, G., Meng, F., Du, D., Wang, S., et al. (2020). Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 11, 216–228.
- Matthay, M.A., Pati, S., and Lee, J.-W. (2017). Concise Review: Mesenchymal Stem (Stromal) Cells: Biology and Preclinical Evidence for Therapeutic Potential for Organ Dysfunction Following Trauma or Sepsis. STEM CELLS 35, 316–324.
- Pati, S., Gerber, M.H., Menge, T.D., Wataha, K.A., Zhao, Y., Baumgartner, J.A., Zhao, J., Letourneau, P.A., Huby, M.P., Baer, L.A., et al. (2011). Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One 6, e25171.
- da Silva Meirelles, L., Fontes, A.M., Covas, D.T., and Caplan, A.I. (2009). Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 20, 419–427.
- Wilson, J.G., Liu, K.D., Zhuo, H., Caballero, L., McMillan, M., Fang, X., Cosgrove, K., Vojnik, R., Calfee, C.S., Lee, J.-W., et al. (2015). Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 3, 24–32.
- Witwer, K.W., Van Balkom, B.W.M., Bruno, S., Choo, A., Dominici, M., Gimona, M., Hill, A.F., De Kleijn, D., Koh, M., Lai, R.C., et al. (2019). Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles 8, 1609206.
View all FLARE content
Learn more about research in the Division of Pulmonary and Critical Care Medicine