ECMO for COVID-19 (Part II)
The FLARE Four
- ECMO is an established rescue intervention for ARDS as reviewed in Part I of this FLARE
- While relatively resource intensive, a number of centers have successfully integrated ECMO into their management of COVID-19
- Outcomes after the use of ECMO for COVID-19 are currently unknown, but extrapolation from pre-COVID-19 use would suggest that they are likely to be encouraging
- Complications of ECMO (not unique to COVID-19) include bleeding, issues related to vascular access, and infection
Introduction
ECMO, used since the 1970s, experienced a resurgence during the 2009 H1N1 pandemic and recently has become an established rescue intervention for ARDS. Last night, we discussed the evidence for VV ECMO in ARDS generally, including two randomized controlled trials (EOLIA and CESAR) demonstrating improved blood oxygenation and establishing the role of ECMO as a rescue therapy, as well as preclinical evidence suggesting the utility of ECMO for preventing ventilator-induced lung injury. As such, many centers have successfully employed ECMO in the current outbreak. Tonight, we will explore the use of ECMO in ARDS related to COVID-19.
Subscribe to the latest updates from FLARE Advances in Motion
Has VV ECMO Been Used in COVID-19 Specifically?
Currently, there are only limited published data available about the use of VV ECMO in COVID-19 (see Table 1). Though VV ECMO has been performed in patients with COVID-19, the reports are scattered. The information included about each particular patient, their clinical course and details of ECMO run is often quite limited and varies substantially between reports. Furthermore, some of the larger case series of COVID-19 patients, including those from Richardson and colleagues (Northwell Health in NYC) and Goyal and colleagues (Cornell, NYC), do not include information about ECMO use.
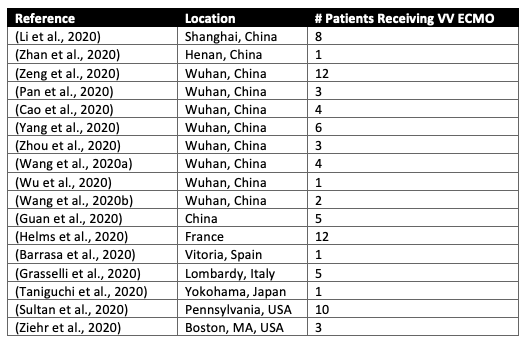
Table 1
Selected published reports of COVID-19 patients receiving VV ECMO drawn from both large case series and case reports.
What Guidance Do We Have on the Use of ECMO in COVID-19?
Two professional societies involved with guidance on the use of ECMO, the Extracorporeal Life Support Organization (ELSO) and the American Society for Artificial Internal Organs (ASAIO), recommend conventional ARDS management, including proning, neuromuscular blockade, and pulmonary vasodilators, prior to initiation of ECMO. The ASAIO recommends escalation to ECMO when other therapies have been maximized and the P:F ratio remains low (Rajagopal Keshava et al., 2020). The ELSO guidance algorithm for COVID-19 ARDS management is below (Bartlett et al., 2020; Ramanathan et al., 2020).
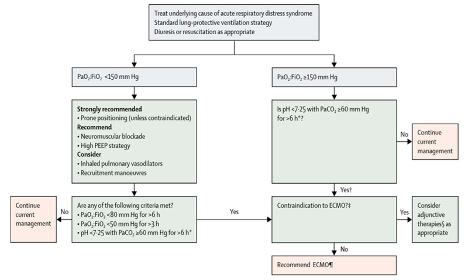
Figure 1
ELSO algorithm for the use of ECMO in COVID-19.
Notably, there are no absolute contraindications for ECMO in the ELSO algorithm except for end-stage respiratory failure when lung transplantation is not an option. Overall, these guidelines are representative of a consensus around the use of ECMO as a rescue intervention after attempts at conventional ventilator management have failed.
Additionally, while not guided by the literature, the femoral-jugular configuration may be preferred for patients with COVID-19 since it does not require guided imaging (i.e., TEE) for cannula placement, and reduces procedures and provider exposure.
What Are Some Complications of VV ECMO?
Major ECMO-related complications include those related to coagulopathy (discussed below), vascular access, infection, and malfunction of the circuit, membrane, and pump. Precise complication rates are difficult to extract since most studies combine both VA and VV ECMO, and are often small, observational, and retrospective.
The most common and feared complications of ECMO are related to coagulopathy, both hemorrhagic and thrombotic. The interface of blood with non-endothelial surfaces (e.g. membranes, pump, cannulas) triggers a pro-inflammatory cascade with hemolytic, thrombocytopenic, hemorrhagic, and thrombotic consequences (Murphy et al., 2015; Thomas et al., 2018). A table of coagulopathic complications from the 2014 ELSO registry of patients on ECMO (both VA or VV) for respiratory failure is shown below (Table 2). Since 2014, significant advances in cannula and membrane development have ameliorated some of the coagulopathic effects, likely reducing complication rates.
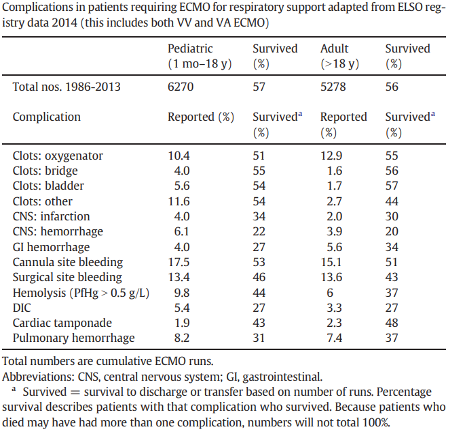
Table 2
Coagulopathic complications in ECMO from ELSO registry (Murphy et al., 2015).
As indicated in the table above, thrombotic complications primarily involve clots affecting the circuit. Thrombosis on the membrane (detected by direct visualization, rising pressure gradient across the membrane, and worsening post-membrane pO2) can impair gas exchange. Overaccumulation of thrombi on the membrane may require a controlled circuit change, whereas thrombosis on the pump itself may require circuit change emergently (Diehl and Gantner, 2018). Whether under controlled or emergent circumstances, this is a high risk procedure given the need to temporarily completely stop blood flow through the circuit and the real risk of introducing air emboli (Sidebotham et al., 2012). Therefore, unless contraindicated, patients on ECMO are almost universally anticoagulated. Some have tried heparin-free ECMO, including in one study of three patients with traumatic brain injuries, all of whom survived and had no thrombotic complications (Muellenbach et al., 2012). However, this is not a common approach.
Thrombosis in the patient is rare, but hemorrhage, likely related both to an underlying coagulopathy (see below) as well as anticoagulation, is common (Thomas et al., 2018). A study of the various hemorrhagic complications in 149 patients on ECMO (111 VA, 38 VV) found that the most common bleeding complications were bleeding at sites of vascular access (37%), hemothorax or cardiac tamponade (17%), ENT bleed (16%), and intracranial hemorrhage (2.2%) (Aubron et al., 2016). However, a retrospective, single-center study of 253 patients on ECMO who were heparinized revealed a 21% rate of intracerebral hemorrhage (Fletcher-Sandersjöö et al., 2017). In COVID-19 specifically, limited ELSO data (Table 3 below) report intracranial hemorrhage in 6% of patients.
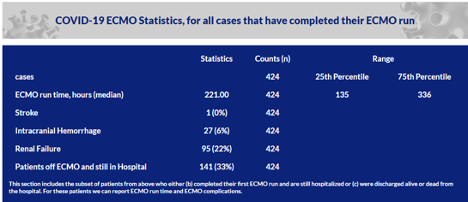
Table 3
Rates of stroke, ICH, and renal failure in patients on ECMO, reported to ELSO.
Several factors are known or thought to contribute to coagulopathy in ECMO:
- Dilutional coagulopathy, when the circuit is primed with crystalloid or red blood cells (Thomas et al., 2018)
- Thrombocytopenia or platelet dysfunction, possibly related to altered glycoprotein Iba levels (Lukito et al., 2016)
- Hyperfibrinolysis from release of tPA from endothelial cells (Hunt et al., 1998)
- Acquired von Willebrand Syndrome, likely caused by shearing of large vWF multimers, can occur within 24 hours of initiating ECMO (Kalbhenn et al., 2015). Recombinant vWF replacement must be considered cautiously, as it can precipitate thrombosis (Hudzik et al., 2015)
- Hemolysis, caused by in-circuit thrombosis, shear stress, and turbulent flow (Appelt et al., 2020)
What Options Exist for Anticoagulation? What Is the Evidence?
In an international survey of 54 institutions, 96% reported using unfractionated heparin as the anticoagulant of choice (Esper et al., 2017), likely because of its rapid onset and easy reversibility. While heparin-induced thrombocytopenia (HIT) is often a concern, one study of 119 patients revealed that despite clinical suspicion of HIT in 19% of patients, it was only truly confirmed in a single patient (Glick et al., 2015). A review of heparin monitoring strategies in 18 studies of VV ECMO used for ARDS including over 640 patients showed significant inter-institutional variability, including between monitoring aPTT and activated clotting time (ACT) for various goal ranges, or antithrombin III levels (Sklar et al., 2016). Given the uncertain reliability of aPTT, some opt to follow anti-Xa levels (Ranucci et al., 2019; Samuel et al., 2016). Alternatively, direct thrombin inhibitors (DTIs) such as bivalirudin or argatroban have been used in some institutions. DTIs are not impacted by relative antithrombin levels and do not induce production of platelet antibodies, but also have limited reversal agents (Burstein et al., 2019; Dingman et al., 2020; Sanfilippo et al., 2017).
Importantly, as our understanding of the presence of coagulation abnormalities in COVID-19 patients evolves (reviewed in April 30 FLARE), the guidance for anticoagulation in ECMO patients may change.
Conclusions
The use of ECMO has been described in viral-associated ARDS, as first established in the H1N1 pandemic of 2009 and more recently in the MERS-CoV outbreak. Limited early data reported in case series and sporadic case reports have suggested that ECMO may be an appropriate intervention in the care of SARS-CoV-2 associated ARDS. The use of ECMO, once other interventions such as proning and inhaled vasodilators have been optimized, carries a weak recommendation supported by low-quality evidence according to the Surviving Sepsis Campaign for COVID-19 (Alhazzani et al., 2020). The WHO clinical guidelines (March 2020) recommend referral to a center with ECMO capacity (in settings where ECMO is available) for patients who have refractory hypoxemia despite lung protective ventilation. Where resources are available, therefore, ECMO is a reasonable option for refractory respiratory failure in COVID-19. Current evidence, while not definitive, suggests that ECMO rescue may be associated with improved outcomes for patients who have failed standard ventilator management.
For interest, here is an article about the experience of one of the first COVID-19 VV ECMO patients cared for at Mass General: 32 Days on a Ventilator.
View all COVID-19 updates
Learn more about research in the Division of Pulmonary and Critical Care Medicine