The second to last HCQ trial?
The FLARE Four
- Initial interest in the use of hydroxychloroquine for the treatment of COVID-19 was spurred by a somewhat plausible mechanism of action and a small study which has since been widely criticized
- While generally well tolerated, hydroxychloroquine is associated with worrisome side effects including cardiac arrhythmia
- Despite a lack of randomized controlled trial data pointing to efficacy, many continue to advocate for the use of hydroxychloroquine. Indeed, the issue of its efficacy has become somewhat polarizing
- An open-label, randomized controlled trial from China shows no benefit of hydroxychloroquine in non-severe COVID-19. The negative result reported here should likely reserve the use of hydroxychloroquine to patients participating in a clinical trial - including the currently enrolling, hopefully definitive PETAL network ORCHID study
Subscribe to the latest updates from FLARE Advances in Motion
Many people are asking...why on earth are we STILL talking about hydroxychloroquine?
Introduction
There is currently only one FDA approved antiviral therapy for SARS-CoV-2, remdesivir, and it has a limited supply. This situation has led to continued interest in unproven treatments including hydroxychloroquine (HCQ). As reviewed in two prior FLAREs (March 22 and April 29), HCQ is known to have in vitro antiviral activity (Liu et al. 2020) and this has led to extensive off-label use during the current pandemic (see table 1). Very little clinical data supports this practice and HCQ has been associated with adverse reactions in pre-print studies when it was used to treat COVID-19 (Ramireddy et al., n.d.; Magagnoli et al. 2020). The FDA has since issued a caution about the use of HCQ outside of a monitored setting. Despite the paucity of evidence for its benefit, HCQ has become unexpectedly politicized (Shear and Haberman 2020), with prominent public figures aggressively advocating its use. This is the context within which we evaluate a newly published randomized controlled trial on the use of HCQ for COVID-19.
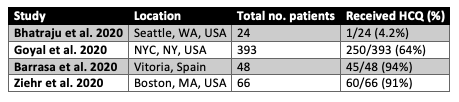
Figure 1
Major case series of COVID-19 patients where hydroxychloroquine use is reported.
The New Study
Tang and colleagues (Tang et al. 2020) report on a multicenter, unblinded, randomized controlled trial of HCQ versus standard of care for patients with mild to moderate COVID-19. This first peer-reviewed, randomized controlled trial of HCQ is informative in a number of respects.
First, the authors frankly describe the difficulties of launching an RCT in the dynamic environment of the pandemic in China. At the first interim analysis conducted by the data safety monitoring board, for example, it was noted that the rapid decline in new cases of COVID-19 meant that the trial was unable to reach its targeted enrollment and it was therefore stopped early. Moreover, at the time of trial termination, only two cases of severe COVID-19 had been randomized and so that arm of the study was not reported. The study is therefore of patients with mild to moderate COVID-19. Mild disease was defined as patients with mild symptoms but no evidence of pneumonia. Moderate disease was defined as patients with fever, cough, sputum production or other symptoms along with radiographic evidence of pneumonia but no hypoxemia defined as SpO2 < 94% on room air or a PaO2:FiO2 ratio of less than 300.
Despite the logistical challenges, the authors were able to complete an informative study. They enrolled 150 pts, 75 in the standard care arm and 75 in the HCQ arm. Patients were well-matched for confounding characteristics and had the typical profile of COVID-19 that has become familiar to readers in multiple case series (Guan et al. 2020; Bhatraju et al. 2020; Ziehr et al. 2020; Grasselli et al. 2020). Mean age was 46, more participants were male (55%), and diabetes and hypertension were common. Of note, roughly a third of patients had been treated with other antiviral agents, but this percentage did not differ significantly between the groups. The treatment arm received a loading dose of HCQ of 1200 mg followed by a maintenance dose of 800 mg daily for up to 14 days. Patients were followed with serial upper and/or lower respiratory tract samples. The primary outcome was probability of PCR conversion (defined as two negative PCR tests more than 24 hrs apart with a cycle threshold* of 40 or more counted as negative) at 28 days. The probability of negative conversion by 28 days (see Fig. 1) in the HCQ group was 85.4% (95% CI 73.8% to 93.8%) versus 81.3% (95% CI 71.2% to 89.6%) in the control group. Adverse events were more common in the HCQ group (30%) than the control group (9%), but none were serious. Two patients in the HCQ group reported adverse events related to disease progression.
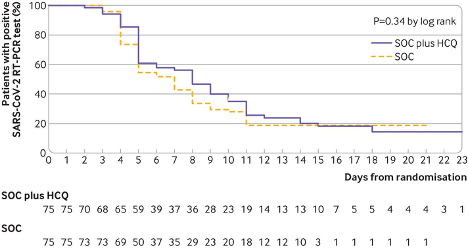
Figure 1
Kaplan-Meier curve for time to negative PCR conversion (Tang et al. 2020).
*not sure what a cycle threshold is? Please see the FLARE on testing for SARS-CoV-2.
What Are We to Make of This Negative Study?
This well conducted, non-blinded, randomized controlled trial contributes to the growing body of evidence that HCQ is ineffective in the treatment of COVID-19. While a small trial is not always definitive, the results of this one should be interpreted in light of the low pretest probability of HCQ utility. While most studies to date are not peer reviewed, the available evidence does not support a major therapeutic effect for HCQ.
Will We Ever Be Able to Stop Talking About HCQ?
Given the enthusiasm for the use of HCQ, it is not clear that there is a level of evidence against it which will convince all of its advocates. That said, the closest thing to a definitive trial we are likely to get is in process -- the Prevention and Early Treatment of Acute Lung Injury Network is currently enrolling patients in the Outcomes Related to COVID-19 Treated with Hydroxychloroquine among In-patients with Symptomatic Disease (ORCHID) Trial. This multicenter, double blinded, randomized control trial will enroll approximately 500 patients. The treatment arm will be HCQ 400mg twice daily as a loading dose, followed by 200mg twice daily for four more days.
The primary outcome is the odds ratio for the change in a 7-point ordinal scale on day 15:
COVID Ordinal Outcomes Scale on Study Day 15:
1. Death
2. Hospitalized on invasive mechanical ventilation or ECMO
3. Hospitalized on non-invasive ventilation or high flow nasal cannula
4. Hospitalized on supplemental oxygen
5. Hospitalized not on supplemental oxygen
6. Not hospitalized with limitation in activity
7. Not hospitalized without limitation in activity
Conclusions
The trial by Tang and colleagues adds to the growing body of evidence that HCQ lacks meaningful activity against SARS-CoV-2. This trial will not end the conversation around HCQ as a more definitive trial is currently enrolling. Given the paucity of positive results, unless and until supportive data from ORCHID are available, HCQ use should be confined to patients enrolled in clinical trials.
References
- Barrasa, Helena, Jordi Rello, Sofia Tejada, Alejandro Martín, Goiatz Balziskueta, Cristina Vinuesa, Borja Fernández-Miret, et al. 2020. “SARS-Cov-2 in Spanish Intensive Care: Early Experience with 15-Day Survival In Vitoria.” Anaesthesia, Critical Care & Pain Medicine, April. https://doi.org/10.1016/j.accpm.2020.04.001.
- Bhatraju, Pavan K., Bijan J. Ghassemieh, Michelle Nichols, Richard Kim, Keith R. Jerome, Arun K. Nalla, Alexander L. Greninger, et al. 2020. “Covid-19 in Critically Ill Patients in the Seattle Region - Case Series.” The New England Journal of Medicine, March. https://doi.org/10.1056/NEJMoa2004500.
- Goyal, Parag, Justin J. Choi, Laura C. Pinheiro, Edward J. Schenck, Ruijun Chen, Assem Jabri, Michael J. Satlin, et al. 2020. “Clinical Characteristics of Covid-19 in New York City.” The New England Journal of Medicine, April. https://doi.org/10.1056/NEJMc2010419.
- Grasselli, Giacomo, Alberto Zangrillo, Alberto Zanella, Massimo Antonelli, Luca Cabrini, Antonio Castelli, Danilo Cereda, et al. 2020. “Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy.” JAMA: The Journal of the American Medical Association, April. https://doi.org/10.1001/jama.2020.5394.
- Guan, Wei-Jie, Zheng-Yi Ni, Yu Hu, Wen-Hua Liang, Chun-Quan Ou, Jian-Xing He, Lei Liu, et al. 2020. “Clinical Characteristics of Coronavirus Disease 2019 in China.” The New England Journal of Medicine 382 (18): 1708–20.
- Liu, Jia, Ruiyuan Cao, Mingyue Xu, Xi Wang, Huanyu Zhang, Hengrui Hu, Yufeng Li, Zhihong Hu, Wu Zhong, and Manli Wang. 2020. “Hydroxychloroquine, a Less Toxic Derivative of Chloroquine, Is Effective in Inhibiting SARS-CoV-2 Infection in Vitro.” Cell Discovery 6 (March): 16.
- Magagnoli, Joseph, Siddharth Narendran, Felipe Pereira, Tammy Cummings, James W. Hardin, S. Scott Sutton, and Jayakrishna Ambati. 2020. “Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized with Covid-19.” Medrxiv. https://www.medrxiv.org/content/10.1101/2020.04.16.20065920v1.abstract.
- Ramireddy, Archana, Harpriya S. Chugh, Kyndaron Reinier, Joseph Ebinger, Eunice Park, Michael Thompson, Eugenio Cingolani, et al. n.d. “Experience with Hydroxychloroquine and Azithromycin in the COVID-19 Pandemic: Implications for QT Interval Monitoring.” https://doi.org/10.1101/2020.04.22.20075671.
- Shear, Michael D., and Maggie Haberman. 2020. “Health Dept. Official Says Doubts on Hydroxychloroquine Led to His Ouster.” The New York Times, April 23, 2020. https://www.nytimes.com/2020/04/22/us/politics/rick-bright-trump-hydroxychloroquine-coronavirus.html.
- Tang, Wei, Zhujun Cao, Mingfeng Han, Zhengyan Wang, Junwen Chen, Wenjin Sun, Yaojie Wu, et al. 2020. “Hydroxychloroquine in Patients with Mainly Mild to Moderate Coronavirus Disease 2019: Open Label, Randomised Controlled Trial.” BMJ 369 (May). https://doi.org/10.1136/bmj.m1849.
- Ziehr, David R., Jehan Alladina, Camille R. Petri, Jason H. Maley, Ari Moskowitz, Benjamin D. Medoff, Kathryn A. Hibbert, B. Taylor Thompson, and C. Corey Hardin. 2020. “Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study.” American Journal of Respiratory and Critical Care Medicine, April. https://doi.org/10.1164/rccm.202004-1163LE.
Learn more about research in the Division of Pulmonary and Critical Care Medicine
View all COVID-19 updates