Pembrolizumab Promising for Patients with Leptomeningeal Carcinomatosis
Key findings
- This open-label, phase 2 trial of pembrolizumab included 20 women who had leptomeningeal dissemination of breast cancer (n=17), non–small cell lung cancer, small cell lung cancer or ovarian cancer
- The trial met its prespecified primary endpoint of at least six patients alive three months after enrollment—12 patients (60%) survived at least that long and median overall survival was 3.6 months
- Best response was stable CNS disease in 11 patients and stable extracranial disease in 10 patients
- Median progression-free survival was 2.6 months for intracranial disease and 3.6 months for extracranial disease
- Eight patients had at least one grade 3/4 adverse event considered at least possibly treatment-related; the only immune-related events were two cases of grade 3 increased aspartate aminotransferase
Some 5%–10% of solid cancers spread to the leptomeninges, and in these cases the prognosis is dismal: survival is about 3–7 weeks. Radiation therapy, sometimes with intrathecal chemotherapy and/or systemic therapy, can extend survival in some patients with leptomeningeal dissemination (LMD), but these treatments have substantial toxicity.
Subscribe to the latest updates from Oncology Advances in Motion
Several clinical trials of immune checkpoint inhibitors have detected high response rates in patients with parenchymal brain metastases from lung cancer and melanoma. Priscilla K. Brastianos, MD, director of the Central Nervous System Metastasis Program at Massachusetts General Hospital Cancer Center; Ryan J. Sullivan, MD, oncologist at Massachusetts General Hospital Cancer Center; and colleagues wondered whether the same could be true for patients with LMD, who have been systematically excluded from trials.
In an open-label phase 2 trial reported in Nature Medicine, they found pembrolizumab to be safe and feasible in this setting.
Study Cohort
Twenty women with LMD of breast cancer (n=17), non–small cell lung cancer, small cell lung cancer or ovarian cancer were enrolled between October 13, 2016, and April 25, 2018. Four breast cancer patients remained on other therapies concurrently with pembrolizumab.
Overall Survival
The prespecified primary endpoint was at least six patients alive three months after enrollment. Twelve patients (60%) survived at least that long and median survival was 3.6 months.
Secondary Outcomes
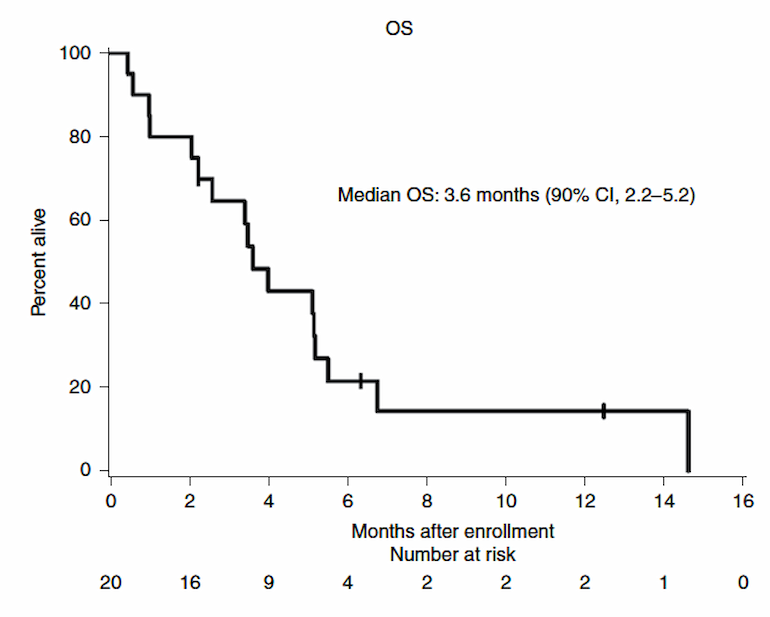
Figure 1
Kaplan-Meier curve in all patients with leptomeningeal disease treated with pembrolizumab (n"="20). Credit: Nature Medicine.
- Response
- CNS disease—11 patients had stable disease as best response, five had progressive disease and four were unevaluable
- Extracranial disease—10 patients had stable disease as best response, one had progressive disease and nine were unevaluable
- Median progression-free survival
- Intracranial disease—2.6 months
- Extracranial disease—3.6 months
Adverse Events
The most frequent adverse reactions (AEs) considered at least possibly related to treatment (TRAEs) were hyperglycemia, nausea and vomiting. Eight patients had at least one grade 3/4 TRAE, most commonly headache (n=3). The only immune-related TRAEs were two cases of grade 3 increased aspartate aminotransferase. This toxicity profile is important because 95% of patients had an ECOG performance status of 0 or 1.
view original journal article Subscription may be required
Learn more about the Mass General Cancer Center
Refer a patient to the Mass General Cancer Center