Genetic Drivers in Hürthle Cell Carcinoma
In This Article
- A Massachusetts General Hospital Cancer Center team has identified important genetic drivers in Hürthle cell carcinoma (HCC): widespread chromosomal losses in the cells and mutations in nuclear and mitochondrial DNA
- HCC accounts for only 3% of thyroid cancers but is a difficult-to-treat tumor
Hürthle cell carcinoma (HCC) accounts for only 3% of thyroid cancers, but those cases present steep challenges. HCC tumors resist the radioactive iodine that is generally used in the detection and treatment of thyroid cancers, and HCC also lacks experimental models, such as cell lines or mice. While previous work has identified genetic factors in HCC, there has been little consensus as to whether these mutations drive cancer progression or are incidental.
Subscribe to the latest updates from Oncology Advances in Motion
In the search for more information, a team led by former Massachusetts General Hospital fellow David McFadden, MD, and including Dora Dias-Santagata, PhD, FACMG, assistant molecular pathologist and co-director of the Translational Research Laboratory at the Mass General Cancer Center, Vamsi Mootha, MD, investigator in the Department of Molecular Biology and Center for Genome Medicine, Gad Getz, PhD, Paul C. Zamecnik Chair in Oncology at the Mass General Cancer Center and director of Bioinformatics at the Mass General Cancer Center and Department of Pathology, as well as director of the Cancer Genome Computational Analysis group at the Broad Institute, Kirsten Kübler, MD, PhD, research fellow at Mass General and the Broad Institute, and Raj K. Gopal, MD, PhD, research fellow in the Department of Molecular Biology, has identified important genetic drivers in HCC: widespread chromosomal losses in the cells, and mutations in nuclear and mitochondrial DNA. Their results were published in the August 2018 issue of Cancer Cell, with Dr. Kübler and Dr. Gopal as joint first authors.
The first factor—widespread chromosomal loss, leading to loss of heterozygosity (LOH)—was especially surprising, and has been observed in the majority of HCC tumors.
"It is certainly something that made us scratch our heads," says Dr. Kübler, noting that this particular type of widespread LOH has not been seen in other thyroid cancers. Dr. Gopal notes that something "cataclysmic" appears to be happening in the nuclear genomes. "Many, many chromosomes are being lost and exist in a single copy state," he says.
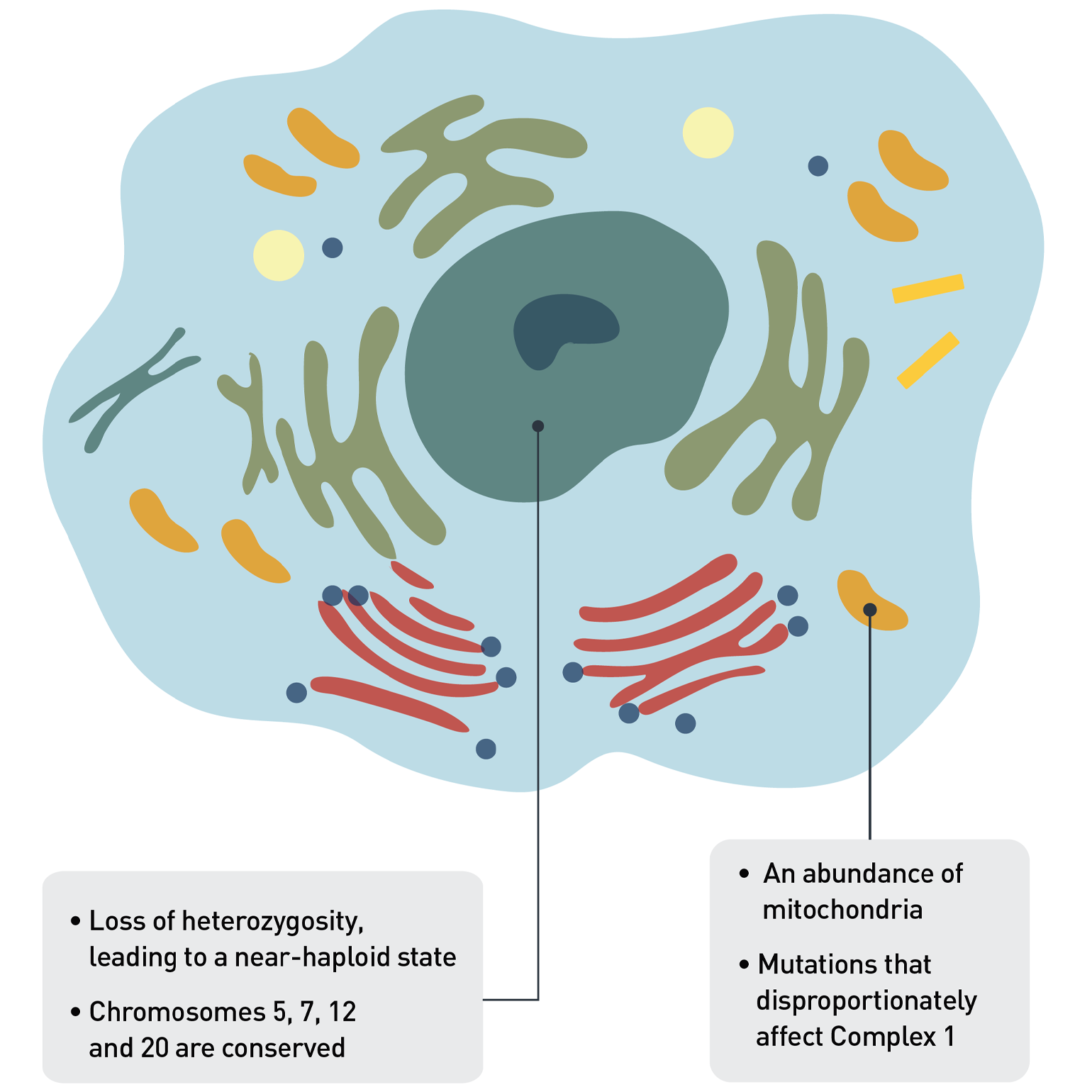
Figure 1. Genetic Anomalies in HCC Cells
Typically, HCC tumors have lost one copy of many of the chromosomes, so that the cell is almost haploid. This is a state which should be unstable, yet HCC seems to thrive even with this widespread loss of chromosomes. HCC cells are also unusual in that they have many more mitochondria than is normal in cancer cells. In addition to nuclear DNA mutations acting as drivers of the disease, mitochondrial DNA mutations in complex I also appear to drive HCC.
Chromosomal Mutation and Near-Haploid State
Widespread chromosomal losses are an early event, says Dr. Gopal, and are maintained even throughout metastasis. This leads to a near-haploid state—in which only one copy of most chromosomes is present—in the majority of tumors they analyzed. This was somewhat surprising given that a near-haploid state is typically assumed to be unstable and therefore generally unfavorable.
"We were all very struck by that result. That finding seemed very robust; we saw it across multiple samples across multiple years. It does not appear to be a transient state. Instead, it appears to be a state that's selected for, that the tumor almost likes," Dr. Gopal says. Why HCC cells not only tolerate, but may even favor, this near-haploid state is not clear but is an area the group is further investigating. Near-haploid states have been seen in cell lines, according to Dr. Kübler, but they are typically not stable.
Despite many genes being lost, both copies of four chromosomes—namely 5, 7, 12 and 20—were typically retained, suggesting some kind of selection in their favor. "There may be something important in those genetic territories, a very important survival gene potentially that the tumors basically cannot lose," Dr. Gopal says. "If we can understand the importance of the gene or genes in these chromosomes, we might leverage that as a therapy."
The group also identified unique mutational drivers in the nuclear DNA of HCC tumors. Besides NRAS, TP53 and the TERT promoter—known thyroid cancer drivers—they found DAXX, NF1, CDKN1A and ARHGAP35 as significantly recurrently altered. The theory that these mutations are drivers is made stronger by the finding that similar mutations occurred across cancer sites.
"We saw convergent evolution in HCC, in which the same genes acquired independent mutations in different tumors from the same patients," says Dr. Kübler, whose area of specialty is genomic alterations. "This further highlights the driver roles of these genes in HCC."
Combined with the lost copies of genes through LOH, these nuclear mutations could completely inactivate genes whose normal function is to suppress cancer.
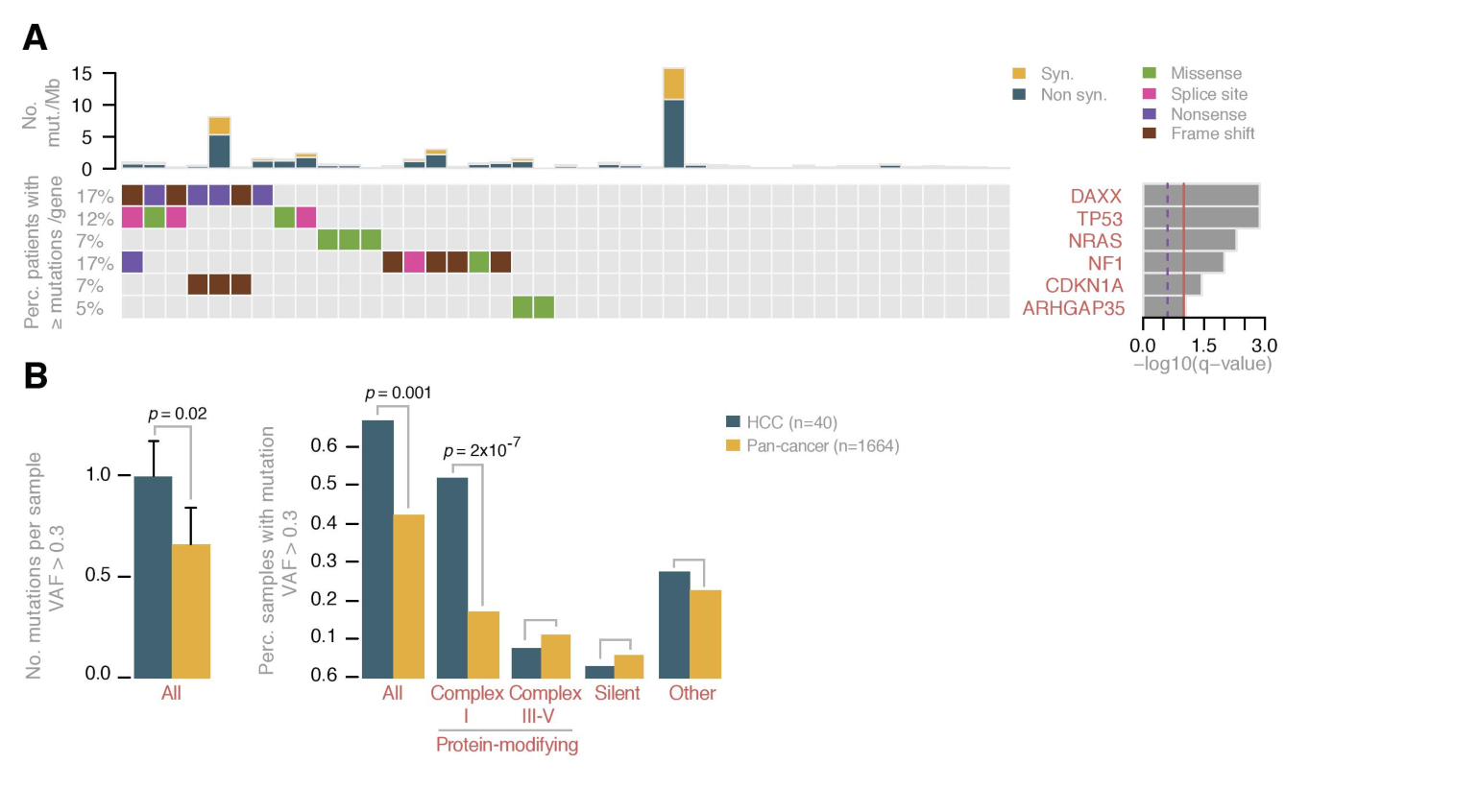
Figure 2. Mutations in HCC
A. Mutation status for nuclear genes in HCC. The matrix shows significantly mutated genes with individual mutations (color-coded by type of mutation). The barplot to the right depicts the q-values.
B. Compared to 31 other cancer types, HCC tumors had more protein-modifying mitochondrial DNA mutations in complex I genes. A breakdown of the mutations seen specifically in the mitochondria in HCC (cyan) and in other cancer types (orange) shows that HCC had more mitochondrial DNA mutations than the other cancer types, and the majority of these mutations were in complex I.
Complex I Mitochondrial DNA Mutations as Unique Drivers
HCC cells are also characterized by an abundance of mitochondria, which are in turn affected by characteristic mutations of their own. The researchers found 29 protein-coding and 17 non-coding mutations in the mitochondrial genome, and their distribution does not appear to be random. Many occurred in genes that encode complex I of the electron transport system, the first of five complexes that culminate in the release of energy from the mitochondria into the cell. Complex I can play a key role in impacting the overall rate of respiration and plays a central role in energy metabolism. Complex I mutations happen early and are maintained as the tumor evolves.
Based on the data they collected, the authors believe that complex I mitochondrial DNA mutations may be driving the growth of HCC cancer cells. First, when they compared the mitochondrial DNA of HCC to 31 other cancer types, they found a far greater prevalence of protein-modifying mutations within complex I genes. Second, these mutations were much more frequent in complex I than in complex III, IV or V.
"In the non-complex I genes, there are really only a few events that occur there, versus complex I gene mutations, which occurred in 60% of patients," says Dr. Gopal, who specializes in the study of mitochondria. Finally, in HCC, these mutations tended to occur in highly conserved areas, hinting that they are likely to impact complex I function.
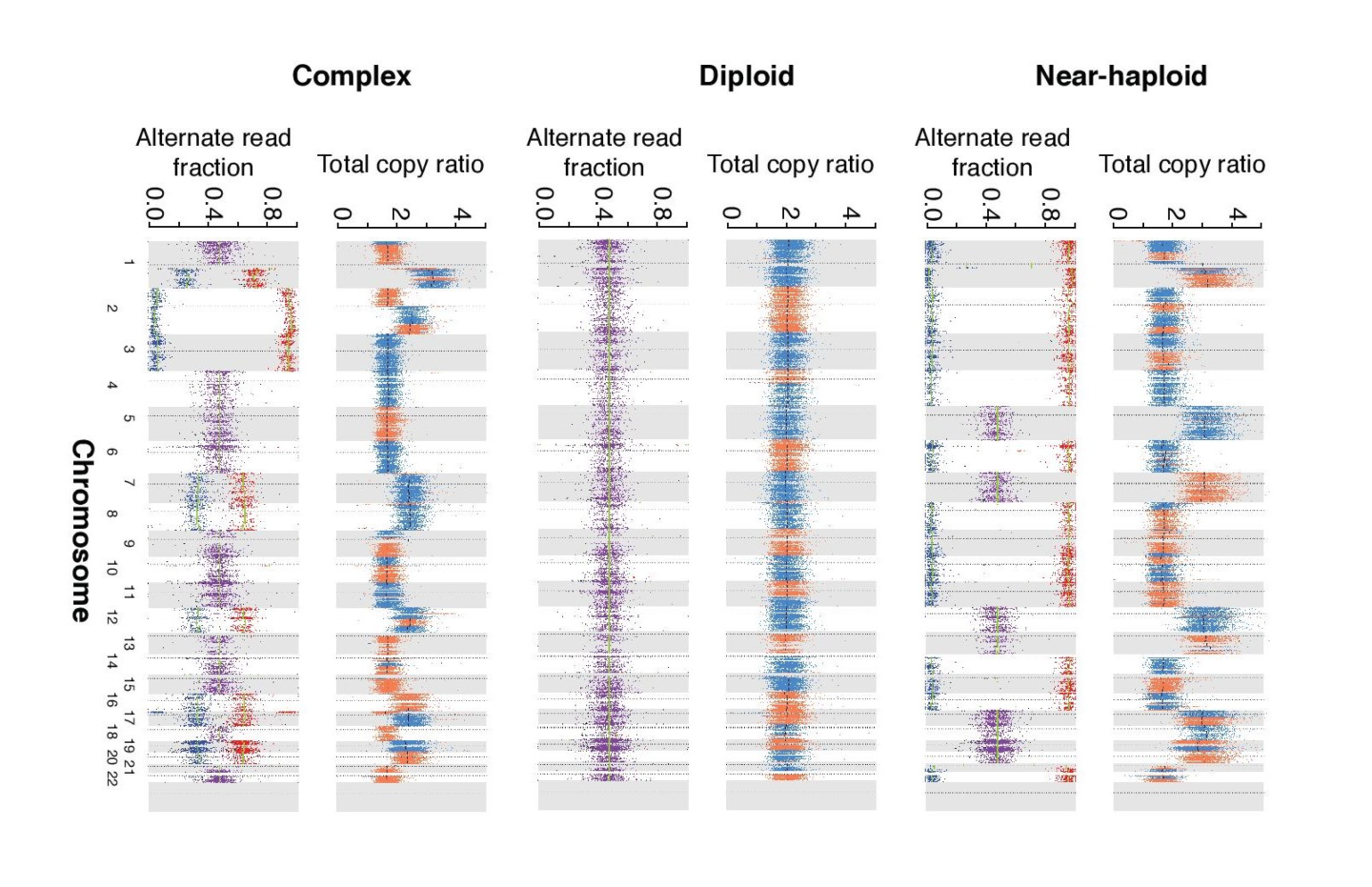
Figure 3. Ploidy in HCC Tumor Cells
Copy-number ratios (upper panel), and allelic ratios of SNVs (lower panel; purple = heterozygous SNVs, red = higher allelic ratio and blue = lower allelic ratio) for three representative tumor samples with near-haploid, diploid and complex chromosomal content.
The mechanisms by which these mitochondrial DNA mutations actually confer an advantage on cells bearing them, however, remains to be determined. While functional targets for treatments may yet be a few steps down the road, the researchers hope that this study may prove key in creating models of HCC and, ultimately, treatments.
"Really this paper is the springboard," says Dr. Gopal, "and we can now start to build a more focused, deeper research enterprise that tries to create primary cell lines from Hürthle patients and mouse models to identify vulnerabilities and, ultimately, new therapies.
The researchers received the Douglass Family Foundation Prize 2018, which honors their research published in the journal Cancer Cell. The award is given annually recognizing the excellent scientific publication from the Mass General Cancer Center.
Learn more about research at the Mass General Cancer Center
Refer a patient to the Mass General Cancer Center