Changes in DNA Structure with Aging Restrain Progression of Cancer
Key findings
- This study investigated the molecular underpinnings of DNA structural changes in colorectal tumor cells and normal cells, with a focus on large-scale reorganization of the conventional genome compartments A and B
- A structurally distinct intermediate compartment (compartment I) was identified at the interface between compartments A and B
- Partitioning of the compartments was profoundly compromised in tumors, accompanied by compartment-specific hypomethylation and chromatin changes, and similar shifts were evident in aged non-malignant cells
- Compartmental breakdown was associated with tumor-suppressive transcription in colon cancer and seven of ten other epithelial tumor types
- Determining how the aging process attempts to stop tumors from forming could lead to innovative types of anti-cancer therapies
Broadly speaking, the human genome is divided into an open, transcriptionally active inner layer of DNA (compartment A), where the genes most often used by cells are readily accessible, and a relatively inactive compartment B.
Subscribe to the latest updates from Oncology Advances in Motion
Sarah E. Johnstone, MD, PhD, of the Department of Pathology and Center for Cancer Research at Massachusetts General Hospital, and colleagues have observed reorganization of this DNA structure in colon cancer cells. But remarkably, rather than being associated with pathology, the reorganization seems to have a tumor-suppressive effect.
In Cell, the researchers explain that their findings could inform cancer therapies, as well as earlier diagnosis and better prognostication.
Compartment I
The researchers mapped the three-dimensional structure of the entire genome in the nuclei of cells from colon tumors and normal colons. They discovered an intermediate compartment at the interface between A and B that interacted with both conventional compartments.
In tumors, the partitioning between compartments A and B broke down. In addition, compartment B relocated toward the nucleus, and compartment I shifted its interactions toward compartment B.
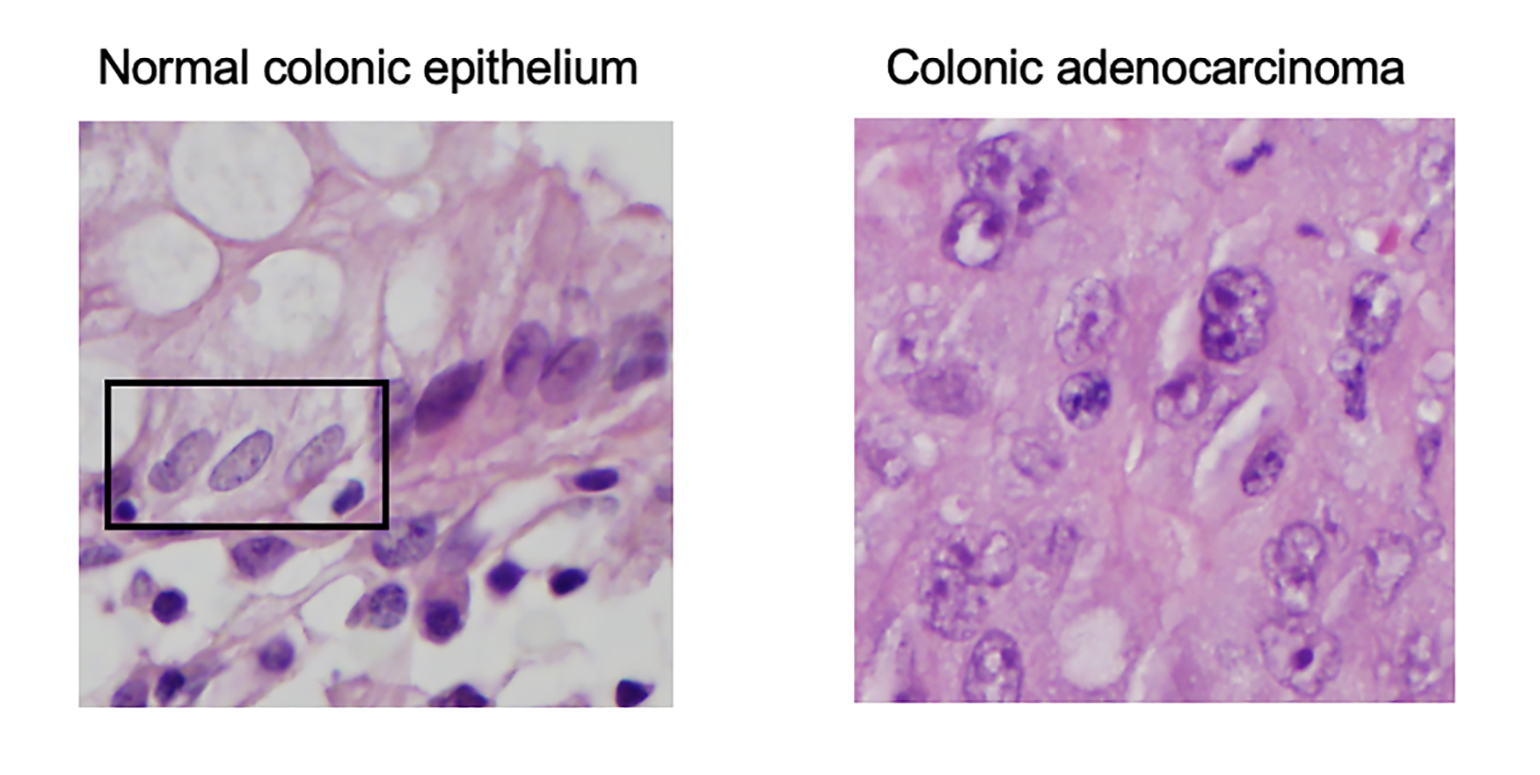
Figure 1
Hematoxylin and eosin stained images for normal colonic epithelial cells (inside box) with pale, even chromatin (LEFT) and colonic adenocarcinoma tumor cells with irregularly shaped, enlarged nuclei with irregular chromatin (RIGHT)
Role of Aging
Compartmental reorganization in tumors correlated with DNA hypomethylation and changes to chromatin loops, another common characteristic of human cancer. Surprisingly, though, artificially aged non-cancerous cells also exhibited those changes. Compartmental reorganization and hypomethylation seem to reflect accumulated cell divisions, not specific oncogenic processes.
Transcriptional Consequences
Despite loss of DNA methylation, the transcriptional activity of compartments B and I was reduced in tumors: 146 genes involved in colorectal cancer progression were downregulated. Additionally, a small number of genes associated with anti-tumor immunity were upregulated. Thus, compartmental breakdown was actually associated with tumor-suppressive transcription.
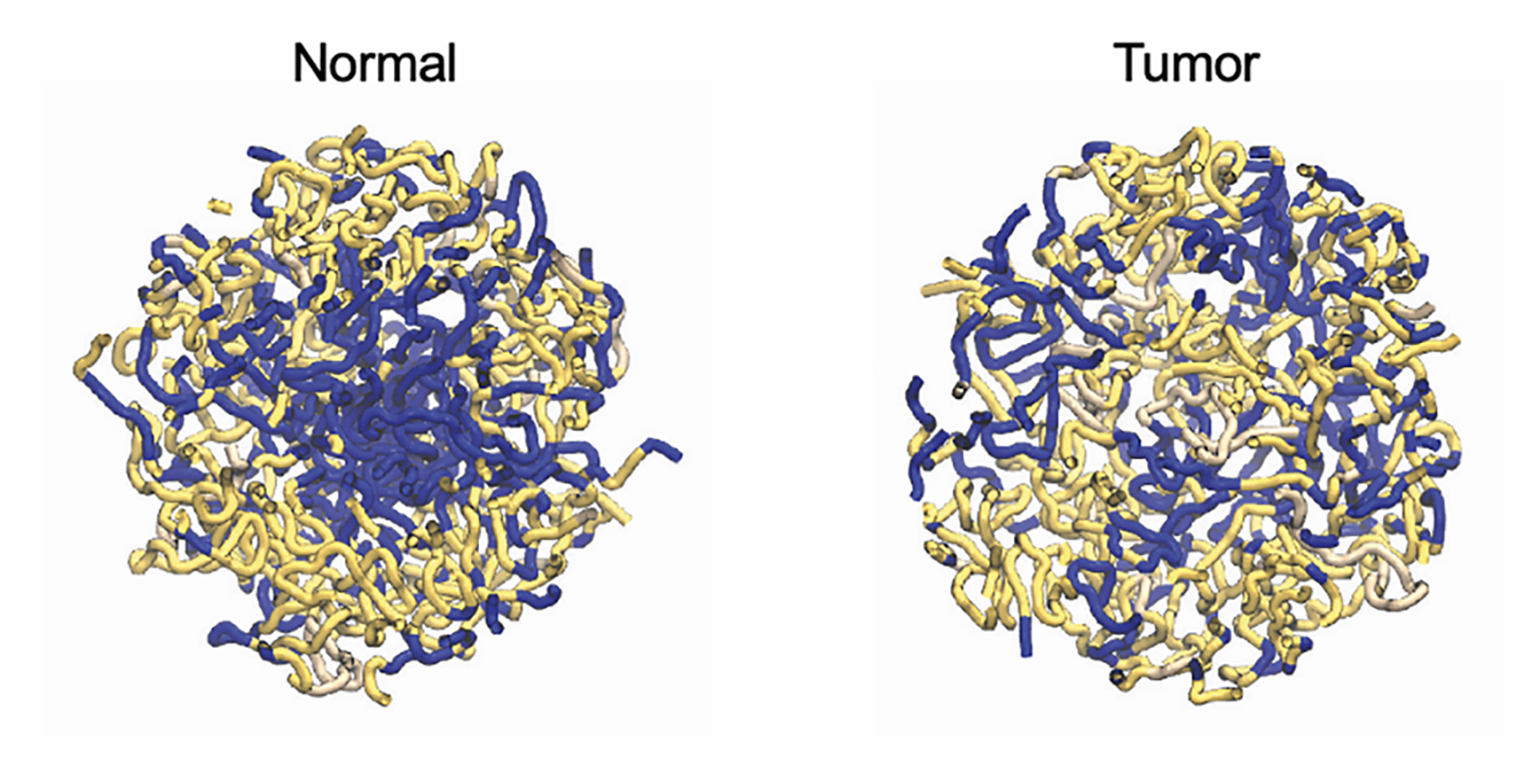
Figure 2
Polymer models for the A (blue) and B (yellow) compartments in both normal colon (LEFT) and colon tumors (RIGHT) shows that tumor nuclei have disorganized segregation of chromatin compartments compared to normal nuclei.
Pan-Cancer Analysis
In seven of ten other epithelial tumor types, a shared set of 367 genes—enriched for oncogenes—was downregulated in association with hypomethylation. This implies that compartmental reorganization might be a general tumor-suppressive mechanism. Determining how the aging process attempts to stop tumors from forming could lead to innovative types of anti-cancer therapies.
view original journal article Subscription may be required
Visit the Center for Cancer Research
Refer a patient to the Mass General Cancer Center