Stromal Microenvironment Shapes the "Architecture" of Pancreatic Tumors, Affecting Survival and Treatment Response
Key findings
- This study investigated the role of cancer-associated fibroblasts (CAFs) on pancreatic ductal carcinoma (PDAC) cell heterogeneity in model systems and translated the findings to primary human tumors
- Dual activation of MAPK and STAT3 was important in generating PDAC cell phenotypes, and this signaling was partly driven by TGF-ß1 produced by CAFs
- Spatial analysis of individual PDAC cells demonstrated that different phenotypes are grouped together in distinct tumor glands in primary human PDAC leading to intratumoral heterogeneity
- Different tumor gland types were associated with differences in clinical outcomes and respond differently to combination chemotherapy
- More accurate assessment of stromal composition and tumor gland spatial analysis might lead to new therapeutic approaches to pancreatic cancer
Subscribe to the latest updates from Oncology Advances in Motion
In the tumor microenvironment of pancreatic ductal adenocarcinoma (PDAC), cancer-associated fibroblasts (CAFs) coexist with normal cells. Recent research has led to contradictory findings that CAFs can either suppress or enhance PDAC growth and metastasis.
David T. Ting, MD, of the Massachusetts General Hospital Cancer Center, and colleagues recently showed that PDAC tumor cell interactions with stromal CAFs are important in intratumoral cellular heterogeneity. In Cell, they describe eight different classes of PDAC tumor glands and explain how the typology correlates with patient outcomes.
Preclinical Findings
The researchers first co-cultured patient-derived PDAC cells and CAFs at various ratios. Confirming a previous study published in Cell Reports by Dr. Ting and others, the researchers observed proliferative (PRO) and invasive epithelial-to-mesenchymal transition (EMT) phenotypes in PDAC cells. They also identified double-positive (DP, PRO+EMT) and double-negative (DN) subpopulations, the latter associated with absence of CAFs.
MAPK and STAT3 pathways were co-activated in DP cells, and CAF-secreted TGF-β1, which engages MAPK signaling in pancreatic cancer, contributed to generating the DP phenotype. Combined MAPK/MEK and STAT3 inhibitors were more effective in abrogating the DP phenotype than either inhibitor alone. TGF-β1 inhibition can also target the development of these DP cells, which is being actively evaluated in the Mass General led SU2C-Lustgarten clinical trial in PDAC.
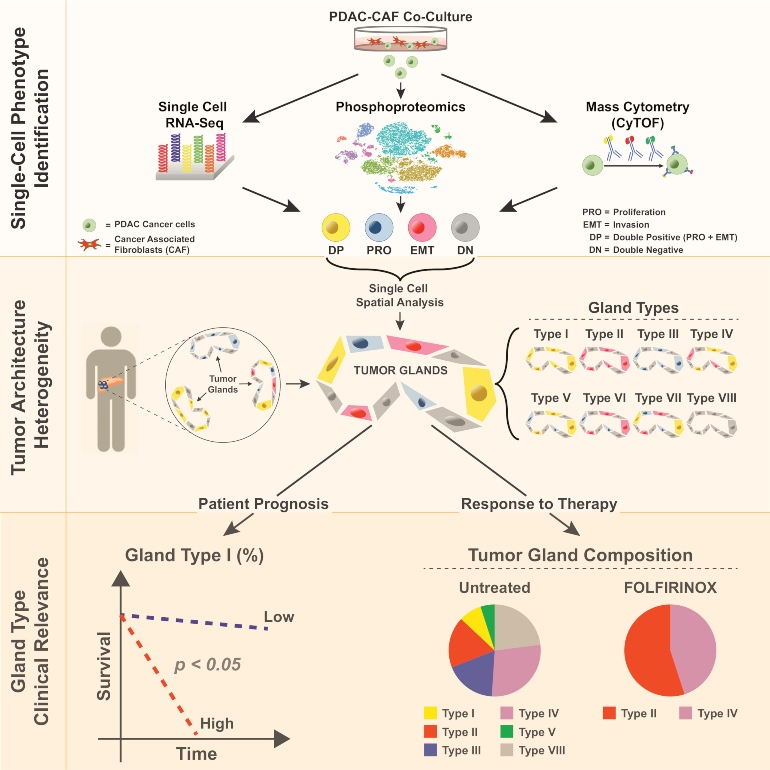
This schema represents the multilayered approach to dissect single cell and tumor gland heterogeneity in pancreatic cancer. Top: From preclinical models we utilized single cell methodologies to understand heterogeneity in a controlled experimental system. Middle: Analysis of single cell phenotypes in patient tumors based on cell culture models identifies that individual tumor cells with different phenotypes can be grouped differently in tumor glands reflecting intratumoral heterogeneity. Bottom: These tumor gland types are then associated with differences in patient outcomes.
Cell Types in Human PDAC
The researchers evaluated each cell type in 319,626 individual cancer cells in 195 human tumors. When normalized by the number of cells per patient (i.e., without spatial information), only PRO cells were prognostic, linked with improved survival.
When cell types were considered part of a unit and normalized on a per-gland basis, DP and DN cells also became significant prognostic markers. In short, a tumor gland carries more information than single cells removed from their "architecture." The implications of this for single-core biopsy remain to be determined.
Distinct Patterns of Tumor Glands
The team defined eight classes of tumor glands, based on cell composition, that were linked to patient outcomes. Examples:
- Liver metastases comprised only EMT- and DP-containing glands
- Glands containing predominantly EMT or DP cells were significantly associated with worse patient survival
- Glands that contained only PRO cells were associated with improved survival
More accurate assessment of stromal composition might lead to new therapeutic approaches to pancreatic cancer. It will also be important to consider variations in stromal composition when evaluating combination drug regimens.
view original journal article Subscription may be required
Learn more about research in the Mass General Cancer Center
Refer a patient to the Mass General Cancer Center