Fighting Zika Virus on Multiple Fronts: Identifying Risk, Improving Screening and Investigating Pregnant Women's Vaccination Attitudes
In This Article
- Data analysis identifies specific Zika risk groups
- A new evidence-based protocol guides shared decision-making around Zika virus testing, particularly in asymptomatic patients
- Ongoing research into pregnant women’s attitudes about Zika vaccines will guide development for optimal patient adherence
The spread of Zika virus has led to one of the most significant public health crises in history. “Never before in history has there been a situation where a bite from a mosquito could result in a devastating (fetal) malformation,” said Tom Frieden, MD, MPH, director of the Center Centers for Disease Control and Prevention (CDC).
Subscribe to the latest updates from OB/GYN Advances in Motion
The CDC’s ever-changing Zika testing guidelines for pregnant women have been a challenge for obstetric providers since the epidemic began in 2016. The latest CDC recommendations, published in July 2017, reverse a previous mainstay of screening and now recommend that only symptomatic pregnant women undergo testing. This change shifts responsibility to clinicians and patients to decide how to best manage Zika screening with little data to guide decision-making.
Massachusetts General Hospital has led discovery in infection diseases for more than a century, and that leadership continues with the Zika outbreak. These efforts are being led by maternal fetal medicine specialist Ilona T. Goldfarb, MD, and former OB/GYN Department member Laura E. Riley, MD. This work, both at Mass General and with external collaborators, seeks to fight the Zika epidemic on multiple fronts by:
- Guiding decision-making about testing for Zika infection
- Filling knowledge gaps about Zika risks and develop testing protocols
- Understanding pregnant women’s attitudes about Zika vaccines to help guide vaccine development
Streamlining the Testing Process
The current work around Zika testing in the Mass General OB/GYN Department has been informed by the team’s experience and leadership during the 2009 outbreak of H1N1 influenza. The department created a centralized process across their six clinical locations and 60 practitioners to streamline actions and ensure every obstetric patient was treated optimally within the H1N1 guidelines. In the process, the department forged a strong working relationship with the Massachusetts Department of Public Health—a partnership they deem a critical element of their success managing outbreaks.
“This is yet another example of the benefit of a public-private partnership,” says Dr. Riley. “We were able to work with our state Department of Public Health to not only benefit Mass General patients, but also serve as a resource to OB/GYNs outside of the Partners HealthCare system who were less familiar with the rapidly changing guidance.”
“We realized during the H1N1 pandemic that if we could bring everyone together under one umbrella to work closely, we could communicate the guidelines quickly and align our practitioners to contain the outbreak,” explains Dr. Goldfarb. “We picked up where we left off with our colleagues at the Massachusetts Department of Public Health to tackle the Zika virus outbreak.”
Here’s how it works: within the OB/GYN Department, two physician “Zika champions” keep up with the most current Zika science, policy, clinical practice and epidemiology and communicate that to other clinicians through a central protocol. A team of outpatient obstetrical “nurse champions” are on the front line with patients: they take calls, screen patients for risk factors and draw on the centralized information from the physician Zika champions to counsel patients (Figure 1).
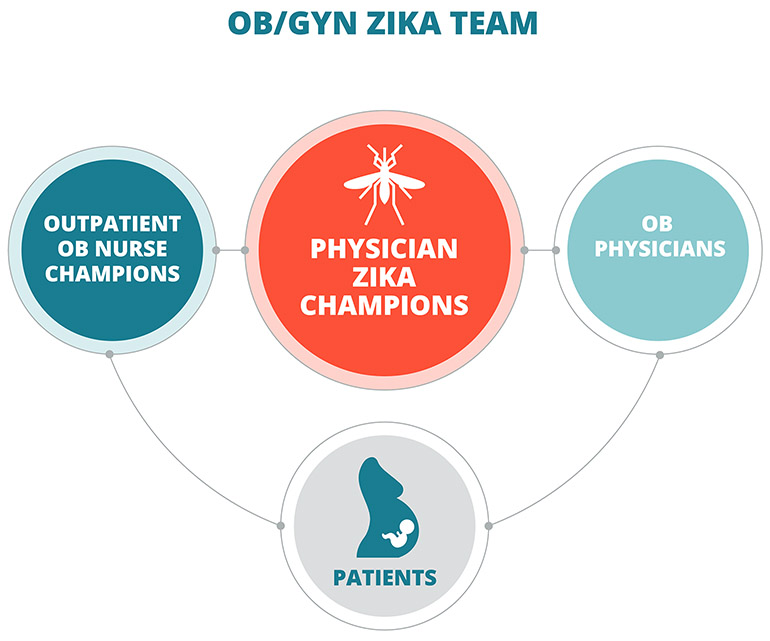
Fig. 1: Mass General Zika Care Team
The Zika care team works collaboratively with both fellow clinicians and patients to ensure that everyone has the information they need.
The Challenge of Asymptomatic Infections
Concerns about Zika-related birth defects continue to create fear in the minds of pregnant women and their care providers, seemingly with good reason. The most recent CDC report published in January 2018 states:
The prevalence of birth defects strongly linked to Zika virus infection increased significantly in areas with local Zika virus transmission (29% more than were expected in the second half of 2016 compared with observed prevalence in the first half). This finding underscores the importance of surveillance for birth defects potentially related to Zika virus infection and the need for continued monitoring in areas at risk for Zika transmission and exposure.
From 2015 to 2016 the Center for Disease Control and Prevention (CDC) required states to test blood serum for all suspected Zika cases regardless of symptoms. Given the imperfections of testing and rising concerns about false positives results, the new interim CDC guidelines recommend Zika testing only if a woman is symptomatic. However, research published in The New England Journal of Medicine has shown that up to 80% of Zika virus-infected individuals do not show symptoms.
Dr. Goldfarb sees this as a public health problem. If a woman has Zika virus but is asymptomatic, she may be missed for screening, and her child will not get the close attention and follow-up that may improve long-term outcomes.
“The asymptomatic aspect of Zika infection is where the confusion comes from,” Dr. Goldfarb explains. “There are big gaps in knowledge about which exposed women are most at risk for infections and which infected women are most at risk of transmitting the infection to their babies. Clinicians are left to decide who to test—and how to counsel women about their risk without data.”
New Testing Guidelines Could Increase Existing Disparities
Dr. Goldfarb expressed this concern in a December 2017 guest editorial in the Journal of the American Medical Association (JAMA), along with colleagues from the University of North Carolina (UNC), Chapel Hill, Anne Drapkin Lyerly, MD, MA, and Elana Jaffe, BA:
The average cost of Zika screening in September 2016 ranged from $229 to $800, according to one report. When testing is not clearly recommended by the CDC, insurance coverage might become a concern, and the extent to which different insurers will cover diagnostic testing for the Zika virus is uncertain. Given that the Zika epidemic is concentrated in lower-resourced populations with historically lower access to quality care, these new national guidelines may increase existing health disparities, as the population most likely to benefit from screening may not receive it.
Identifying a New At-risk Subgroup
To help define rational, effective application of Zika testing, Dr. Goldfarb and Dr. Riley have undertaken more granular data analyses. The team examined the cohort of pregnant women at Mass General exposed to Zika virus from the beginning of the epidemic in 2016 and looked for predictors of a Zika virus infection. This analysis identified a more specific risk subgroup: immigrants or returning travelers who had spent more than a trimester in an area of active Zika transmission were at risk of Zika virus infection with or without reported symptoms of illness.
Armed with this newly defined at-risk group, the Mass General team is helping their clinicians make more informed decisions about which patients to test. As has been the practice in epidemic management, the Zika champions have developed a new protocol to support evidence-based decision-making for Zika testing to guide clinicians and their patients through this process (Figure 2).
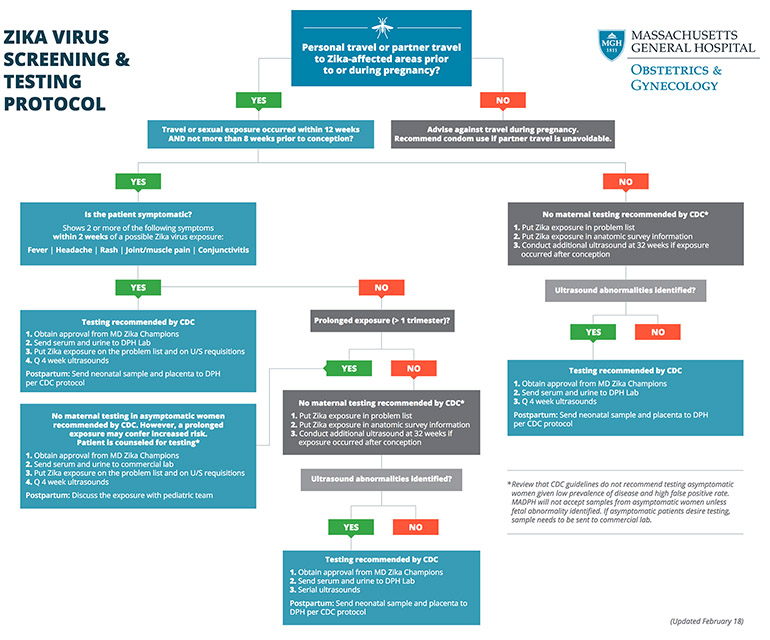
Fig. 2: Mass General Zika Testing Protocol
Mass General Zika Testing Protocol. This protocol guides physicians through the diagnostic process to engage patient in evidence-based collaborative decisions about Zika testing. (See full size PDF)
Looking Forward: Preventing Zika Infections
Several vaccines to prevent Zika infection are in early research and development. Traditionally vaccine testing has excluded pregnant women out of concerns for the health of the mother and fetus. But because the Zika virus poses a serious threat to the long-term health of the fetus, a Zika vaccine’s success hinges on its acceptability to and effectiveness in pregnant women and women of reproductive age.
Dr. Goldfarb and her collaborators at UNC, Dr. Lyerly and Elana Jaffe, believe that pregnant women must be included in these trials. “In this case we feel it’s an ethical issue to include pregnant women when testing Zika vaccines,” says Dr. Goldfarb.
Dr. Goldfarb and Dr. Lyerly are now studying pregnant women’s attitudes and willingness to be enrolled in vaccine trials. The team’s research aims to determine which type of vaccine platforms pregnant women would be most willing to accept. To help determine this, they interviewed over 130 pregnant women.
The data are still being analyzed, but Dr. Goldfarb and team note an emerging trend: pregnant women support the concept of including pregnant women in Zika vaccine research and are motivated to participate by a desire to protect their own health and their baby’s health, as well as that of the public from a Zika pandemic.
Though there is still work to be done, Dr. Goldfarb knows this much: “Zika is on the radar now and will remain there. We’re doing all that we can to be ready for it as the outbreak ebbs and flows.”
Learn more about the Obstetrics Program
Explore OB/GYN research at Mass General