Superinfection in COVID-19
The FLARE Four
- Secondary bacterial infection has been reported to be a major cause of mortality in viral respiratory infections prior to COVID-19
- Available data on secondary infections in COVID-19 are limited but do indicate that nosocomial infections are associated with increased COVID-19 severity and death
- Risk factors for secondary bacterial and fungal infections include invasive devices (central venous catheters), diabetes, combination antibiotic therapy, and glucocorticoid treatment
- Given long ICU stays associated with COVID-19, it is likely that rates of secondary bacterial infection will reflect, in part, nosocomial infections that are common in critical illness
Many people are saying...bacterial superinfection is a driver of mortality in viral pneumonia such as influenza and COVID-19.
Subscribe to the latest updates from FLARE Advances in Motion
Introduction
Although SARS-CoV-2 infection is responsible for COVID-19, it remains unclear whether viral-induced cell death per se is ultimately fully responsible for the clinical syndrome following infection. Prior FLAREs have discussed the possibility of “dysregulated immunity” as a potential contributor, but another important potential source of morbidity is co-infection with other pathogens - bacterial, fungal, and viral alike. Indeed, Fauci and colleagues (yes, that Fauci) noted that most patients who died during the 1918 influenza pandemic had autopsy evidence of bacterial pneumonia (Morens, Taubenberger, and Fauci 2008), a finding recognized even by contemporary physicians - one of whom noted in 1919, “If grippe (influenza) condemns, the secondary infections execute” (Cruveilhier 1919).
Multiple reports have identified co-infections in COVID-19 patients, but the epidemiology and pathological consequences of these remain ill-defined. In addition, many such infections might simply reflect prolonged hospitalizations and its attendant risks rather than factors unique to the virology of SARS-CoV-2. In this FLARE, we explore the risk factors associated with secondary infection, the role of prophylactic antimicrobial therapy, and approaches to mitigating nosocomial infections in patients with COVID-19.
Why Are People So Concerned About Bacterial Infections if COVID-19 Is a Viral Illness?
Laboratory models have established that viral infection may disrupt host mucociliary clearance and immune response, decreasing the threshold for bacterial infection (Hendaus, Jomha, and Alhammadi 2015). Furthermore, observations such as those by Fauci and colleagues have led many to posit that secondary infection is a major driver of morbidity and mortality in viral respiratory disease. In addition to data from the 1918 influenza pandemic, bacterial superinfection was reported to be associated with poor outcome in the 1957 H2N2 and the 1968 H3N2 influenza pandemics (Kash and Taubenberger 2015). This association persists even in the modern antibiotic era. During the 2009 outbreak of severe respiratory failure secondary to H1N1 influenza (Rice et al. 2012), 30% of critically ill patients were diagnosed with bacteremia or bacterial pneumonia within 72 hours of ICU presentation - an observation consistent with the idea that bacterial superinfection was a significant driver of severe disease. Indeed, compared to patients who were not diagnosed with bacterial infection, these patients were more likely to develop shock, require extended ICU care, mechanical ventilation, and die.
Lessons From Pediatric Respiratory Failure
The published data about viral co-infection in COVID-19 patients suffer from inconsistencies. One report from the San Francisco Bay area examined symptomatic patients with likely respiratory viral illness and identified just ~10% (total 116) positive for SARS-CoV-2, of which 20% were also PCR positive for another pathogen. These data suggested a substantial incidence of viral co-infection among COVID-19 patients (Kim et al. 2020). However, another study from the same region at the same time tested 166 hospitalized COVID-19 patients for influenza/RSV and found that none were positive for these pathogens (Myers et al. 2020). Similarly, a Wuhan cohort of 99 inpatients revealed zero viral co-infections (Chen et al. 2020). How can we reconcile these data? One possible explanation might lie in the now well-recognized rate of asymptomatic SARS-CoV-2 infection, such that many positive tests in non-hospitalized individuals in the first study (Kim et al. 2020) may represent incidental findings rather than drivers of URI symptoms.
Much more granular data are available regarding bacterial co-infection, particularly in patients hospitalized with moderate to severe COVID-19 (Ruan et al. 2020; X. Yang et al. 2020; Wang et al. 2020; Zhang et al. 2020; Chen et al. 2020). A retrospective study of 918 COVID-19 patients from Wuhan, China found that 7.1% had a bacterial or fungal co-infection (He et al. 2020). The most common types were pneumonia (32.3%), bacteremia (24.6%) and urinary tract infection (21.5%). Two smaller cohort studies have reported similar nosocomial infection rates (8% of 150 hospitalized patients (Ruan et al. 2020) and 13.5% of 52 mechanically ventilated patients (X. Yang et al. 2020). In contrast, one study of 339 COVID-19 patients over 60 years of age with severe and critical disease were found to have bacterial secondary infection in 42.8% of cases (Wang et al. 2020). The table below summarizes these and related data.
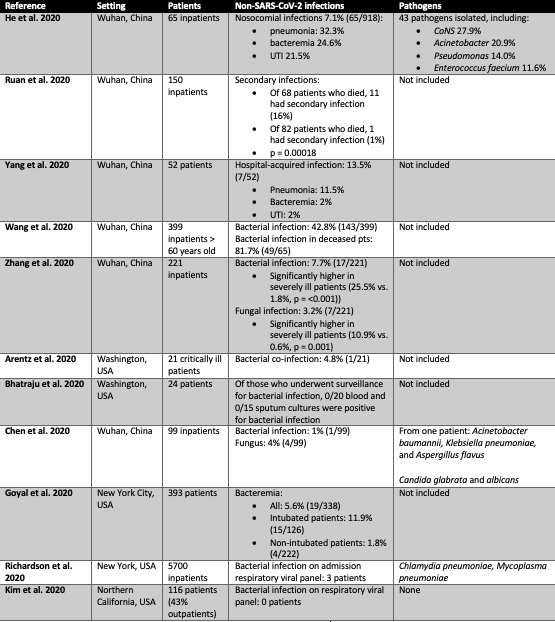
Table 1
Comparison of major studies reporting bacterial and fungal infections in patients with SARS-CoV-2 infection. Readers should note that some hospital systems utilize a respiratory virus panel which includes PCR testing for two bacterial pathogens, C. pneumoniae and M. pneumoniae. The individual pathogens tested for in these panels are not consistently described by authors across these studies.
What Are the Organisms?
Specific organisms have not been typically reported (see Table). Fungal pathogens such as Candida albicans, Aspergillus species, Pneumocystis jirovecii, and Mucor species have been described in a small subset of patients and case reports (He et al. 2020; X. Yang et al. 2020; Wang et al. 2020; Zhang et al. 2020; Chen et al. 2020).
Notably, many infections reported in those with COVID-19 are caused by drug-resistant organisms. These include Pseudomonas aeruginosa, ESBL Klebsiella pneumoniae, multidrug resistant E. coli, Acinetobacter, and Enterococcus (He et al. 2020; X. Yang et al. 2020; Wang et al. 2020; Zhang et al. 2020; Chen et al. 2020).
What Risk Factors Might Account for Secondary Infection?
In a single center study of 65 COVID-19 patients who developed a nosocomial infection, invasive devices (OR 4.28, 95% CI: 2.47–8.61), diabetes (OR 3.06, 95% CI: 1.41–7.22), and use of more than one class of antibiotic (OR 1.84, 95% CI: 1.31–4.59) were significant predictors of nosocomial infection (He et al. 2020). In this study, glucocorticoid use was also positively associated with secondary infection with 38% of patients exposed to glucocorticoids. Interestingly, 75.4% of patients who developed secondary bacterial and/or fungal infection were receiving prophylactic antibiotics. A disproportionately high use of antibiotics has been reported in people with COVID-19, even with an overall low incidence of known bacterial infections (Zhou et al. 2020).
In a meta-analysis of 5270 patients with coronaviruses, including SARS-CoV-1, MERS-CoV and SARS-CoV-2, treatment with corticosteroids was strongly associated with bacterial infection (RR = 2.08, 95% CI 1.54-2.81, p <0.001), though this only examined patients from two studies where occurrence of bacterial infection was reported (Z. Yang et al. 2020). Pooled mortality from these studies indicated an increased risk of death in association with steroids (RR = 2.11, 95%CI = 1.13–3.94, P = 0.019), though patients with severe disease were also more likely to receive corticosteroids.
In the pre COVID-19 era, there are differing reports of the effect of corticosteroids on rates of infection in large patient cohorts. In the CORTICUS trial, a randomized trial of hydrocortisone for relative adrenal insufficiency in septic shock, there was a statistically significant increase (odds ratio 1.37 (95% CI, 1.05 to 1.79) in superinfection after receiving hydrocortisone (Sprung et al. 2008). However, in the ADRENAL trial, 2800 patients were randomized to hydrocortisone or placebo for septic shock. There was no difference in the occurrence of new bacteremia or fungemia between groups (14.1% in both groups) (Venkatesh et al. 2018). Thus the data are mixed as to the effect of steroids on the risk of infection and may be pathogen-dependent.
What Is the Role of Typical Hospital Acquired Infections?
Some bacterial infections may be more related to long hospitalization rather than a complication of bacterial pneumonia per se. There is a strong association between nosocomial infection and mortality (He et al. 2020; Wang et al. 2020). In a pre-print, non-peer reviewed study, Zhang et al. evaluated the differences in secondary infection between those with non-severe COVID-19 and those with severe disease (Zhang et al. 2020). As expected, patients with severe COVID-19 suffered a significantly higher rate of secondary infection (see Table above). Another study showed a (perhaps predictable) association between indwelling catheters and nosocomial infection with an odds ratio of 4.28 (CI: 2.47-8.61) (He et al. 2020). In this study, the most common pathogen reported was coagulase-negative Staphylococcus.
Are Empiric Antibiotics or Antifungal Treatments Something We Should Consider?
COVID-19 patients with secondary infection have poor outcomes with higher risk of mortality. However as highlighted above (He et al. 2020), 75.4% of patients who developed secondary bacterial or fungal infections were already receiving prophylactic antibiotics. Regimens included fluoroquinolones (61.5%), combination of antibiotics (not otherwise defined, 10.8%) and cephalosporins (9.2%). This suggests that prophylactic agents may not prevent the hospital-acquired infections and risk selecting for more drug-resistant pathogens.
Others have postulated that critically ill COVID-19 patients are at risk for invasive fungal diseases based on a previously described association between influenza and invasive aspergillosis (Gangneux et al. 2020; Vanderbeke et al. 2018). The theoretical pathophysiology is thought to be twofold: 1. viral destruction of bronchial epithelium allowing an opportunity for the colonizing fungi to cause invasive disease and 2. SARS-CoV-2 induced lymphopenia. More data are needed to investigate how this immune dysregulation influences the risk for secondary bacterial and fungal infections.
Conclusions
The secondary infections observed in COVID-19 patients are a consequence of viral pneumonia but also prolonged hospital exposure and critical illness. Nosocomial infections related to long hospital course impact severity of COVID-19 and increase mortality. Indwelling devices, diabetes, prophylactic antibiotics, and glucocorticoids have been associated with increased secondary infection risk. Many of the pathogenic organisms are multidrug-resistant, hospital-acquired infections; therefore, we recommend a low threshold to obtain culture data in COVID-19 patients and the use of appropriately targeted antibiotic therapy for this population.
References:
- Arentz, Matt, Eric Yim, Lindy Klaff, Sharukh Lokhandwala, Francis X. Riedo, Maria Chong, and Melissa Lee. 2020. “Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State.” JAMA: The Journal of the American Medical Association. https://doi.org/10.1001/jama.2020.4326.
- Bhatraju, Pavan K., Bijan J. Ghassemieh, Michelle Nichols, Richard Kim, Keith R. Jerome, Arun K. Nalla, Alexander L. Greninger, et al. 2020. “Covid-19 in Critically Ill Patients in the Seattle Region—case Series.” The New England Journal of Medicine. https://www.nejm.org/doi/full/10.1056/NEJMoa2004500.
- Chen, Nanshan, Min Zhou, Xuan Dong, Jieming Qu, Fengyun Gong, Yang Han, Yang Qiu, et al. 2020. “Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study.” The Lancet 395 (10223): 507–13.
- Cruveilhier, Louis. 1919. “Action Du Sérum Antipneumococcique Au Cours de La Pneumonie et Dans Les Complications de La Grippe.” In Annales de l’Institut Pasteur, 33:448–61.
- Gangneux, J-P, M-E Bougnoux, E. Dannaoui, M. Cornet, and J. R. Zahar. 2020. “Invasive Fungal Diseases during COVID-19: We Should Be Prepared.” Journal de Mycologie Medicale, April, 100971.
- Goyal, Parag, Justin J. Choi, Laura C. Pinheiro, Edward J. Schenck, Ruijun Chen, Assem Jabri, Michael J. Satlin, et al. 2020. “Clinical Characteristics of Covid-19 in New York City.” The New England Journal of Medicine, April. https://doi.org/10.1056/NEJMc2010419.
- Hendaus, Mohamed A., Fatima A. Jomha, and Ahmed H. Alhammadi. 2015. “Virus-Induced Secondary Bacterial Infection: A Concise Review.” Therapeutics and Clinical Risk Management 11 (August): 1265–71.
- He, Yan, Wei Li, Zhen Wang, Huilong Chen, Lei Tian, and Dong Liu. 2020. “Nosocomial Infection among Patients with COVID-19: A Retrospective Data Analysis of 918 Cases from a Single Center in Wuhan, China.” Infection Control and Hospital Epidemiology: The Official Journal of the Society of Hospital Epidemiologists of America, April, 1–2.
- Kash, John C., and Jeffery K. Taubenberger. 2015. “The Role of Viral, Host, and Secondary Bacterial Factors in Influenza Pathogenesis.” The American Journal of Pathology 185 (6): 1528–36.
- Kim, David, James Quinn, Benjamin Pinsky, Nigam H. Shah, and Ian Brown. 2020. “Rates of Co-Infection Between SARS-CoV-2 and Other Respiratory Pathogens.” JAMA: The Journal of the American Medical Association, April. https://doi.org/10.1001/jama.2020.6266.
- Morens, David M., Jeffery K. Taubenberger, and Anthony S. Fauci. 2008. “Predominant Role of Bacterial Pneumonia as a Cause of Death in Pandemic Influenza: Implications for Pandemic Influenza Preparedness.” The Journal of Infectious Diseases 198 (7): 962–70.
- Myers, Laura C., Stephen M. Parodi, Gabriel J. Escobar, and Vincent X. Liu. 2020. “Characteristics of Hospitalized Adults With COVID-19 in an Integrated Health Care System in California.” JAMA: The Journal of the American Medical Association, April. https://doi.org/10.1001/jama.2020.7202.
- Rice, Todd W., Lewis Rubinson, Timothy M. Uyeki, Frances L. Vaughn, Benjamin B. John, Russell R. Miller 3rd, Elizabeth Higgs, et al. 2012. “Critical Illness from 2009 Pandemic Influenza A Virus and Bacterial Coinfection in the United States.” Critical Care Medicine 40 (5): 1487–98.
- Richardson, Safiya, Jamie S. Hirsch, Mangala Narasimhan, James M. Crawford, Thomas McGinn, Karina W. Davidson, Douglas P. Barnaby, et al. 2020. “Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area.” JAMA: The Journal of the American Medical Association, April. https://doi.org/10.1001/jama.2020.6775.
- Ruan, Qiurong, Kun Yang, Wenxia Wang, Lingyu Jiang, and Jianxin Song. 2020. “Correction to: Clinical Predictors of Mortality due to COVID-19 Based on an Analysis of Data of 150 Patients from Wuhan, China.” Intensive Care Medicine, April. https://doi.org/10.1007/s00134-020-06028-z.
- Sprung, Charles L., Djillali Annane, Didier Keh, Rui Moreno, Mervyn Singer, Klaus Freivogel, Yoram G. Weiss, et al. 2008. “Hydrocortisone Therapy for Patients with Septic Shock.” The New England Journal of Medicine 358 (2): 111–24.
- Vanderbeke, Lore, Isabel Spriet, Christine Breynaert, Bart J. A. Rijnders, Paul E. Verweij, and Joost Wauters. 2018. “Invasive Pulmonary Aspergillosis Complicating Severe Influenza: Epidemiology, Diagnosis and Treatment.” Current Opinion in Infectious Diseases 31 (6): 471–80.
- Venkatesh, Balasubramanian, Simon Finfer, Jeremy Cohen, Dorrilyn Rajbhandari, Yaseen Arabi, Rinaldo Bellomo, Laurent Billot, et al. 2018. “Adjunctive Glucocorticoid Therapy in Patients with Septic Shock.” The New England Journal of Medicine 378 (9): 797–808.
- Wang, Lang, Wenbo He, Xiaomei Yu, Dalong Hu, Mingwei Bao, Huafen Liu, Jiali Zhou, and Hong Jiang. 2020. “Coronavirus Disease 2019 in Elderly Patients: Characteristics and Prognostic Factors Based on 4-Week Follow-Up.” The Journal of Infection, March. https://doi.org/10.1016/j.jinf.2020.03.019.
- Yang, Xiaobo, Yuan Yu, Jiqian Xu, Huaqing Shu, Jia ’an Xia, Hong Liu, Yongran Wu, et al. 2020. “Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study.” The Lancet. Respiratory Medicine, February. https://doi.org/10.1016/S2213-2600(20)30079-5.
- Yang, Zhenwei, Jialong Liu, Yunjiao Zhou, Xixian Zhao, Qiu Zhao, and Jing Liu. 2020. “The Effect of Corticosteroid Treatment on Patients with Coronavirus Infection: A Systematic Review and Meta-Analysis.” The Journal of Infection, April. https://doi.org/10.1016/j.jinf.2020.03.062.
- Zhang, Guqin, Chang Hu, Linjie Luo, Fang Fang, Yongfeng Chen, Jianguo Li, Zhiyong Peng, and Huaqin Pan. 2020. “Clinical Features and Outcomes of 221 Patients with COVID-19 in Wuhan, China.” Respiratory Medicine. medRxiv. https://doi.org/10.1101/2020.03.02.20030452.
- Zhou, Pengcheng, Zhenguo Liu, Yuhua Chen, Yinzong Xiao, Xun Huang, and Xue-Gong Fan. 2020. “Bacterial and Fungal Infections in COVID-19 Patients: A Matter of Concern.” Infection Control and Hospital Epidemiology: The Official Journal of the Society of Hospital Epidemiologists of America, April, 1–7.
View all FLARE content
Learn more about research in the Division of Pulmonary and Critical Care Medicine