Anticoagulation in COVID-19
The FLARE Four
- Increased activation of the coagulation cascade and reduced fibrinolytic activity are observed in ARDS, but no human trial in ARDS has demonstrated a clinical benefit to therapeutic anticoagulation
- Observational data and case reports have suggested a high rate of thrombotic complications in COVID-19, particularly in those with ARDS
- These data have generated excitement and controversy regarding empiric systemic anticoagulation and alternative therapies (including tissue plasminogen activator and post-discharge thromboprophylaxis) in severe COVID-19
- Guideline data support the use of thromboprophylaxis in acutely ill and critically ill patients with COVID-19 without bleeding contraindications. The utility of augmented anticoagulation strategies in the critically ill COVID-19 population is presently unknown
Many people are saying...we should give therapeutic anticoagulation to patients admitted with COVID-19.
Subscribe to the latest updates from FLARE Advances in Motion
Introduction
Severe COVID-19 is often associated with dramatically elevated D-Dimer and fibrinogen. In this FLARE, we review the incidence of clotting in COVID-19 patients and discuss proposed strategies for anticoagulation.
ARDS, Critical Illness, Coagulopathy and Anticoagulation
While much attention has been paid to elevated indices of coagulation, thrombosis and possible microvascular dysfunction in COVID-19, it is worth remembering that ARDS has long been known to be associated with coagulopathy, thrombosis and microvascular dysfunction. Coagulopathy in ARDS is thought to involve alveolar fibrin deposition, depressed fibrinolysis and activation of coagulation pathways by damage to the alveolar endothelium leading to exposure of tissue factor (Idell 2003). Endothelial damage and coagulopathy in ARDS have been associated with both macrovascular (Greene et al. 1987) and microvascular thrombosis (Ospina-Tascón et al. 2020), elevated dead space fraction, shunt and hypoxemia (Snow et al. 1982). A retrospective review of ARDS in association with H1N1 influenza reported a 9% incidence of venous thrombosis (Rice et al. 2012).
Beyond ARDS, critically ill patients in general have a higher risk of thrombosis. In a retrospective review of 355 critically ill patients, Hanify et al. reported that 12.5% developed VTE despite pharmacologic prophylaxis, with ARDS shown to be an independent risk factor (Hanify et al. 2017). Many inhibitors of the coagulation pathway have been tested in animal and human models in ARDS, including nebulized and intravenous heparin, antithrombin, tissue factor pathway inhibitor, plasminogen activators, activated protein C, and thrombomodulin. No trial in humans to date has reported a significant clinical benefit in ARDS (Camprubí-Rimblas et al. 2018). The March 31, 2020 FLARE “Coagulation and ARDS in COVID-19” summarizes the relationship between hypercoagulability and ARDS and highlights the absence of convincing evidence to support anticoagulation in ARDS.
What Do We Know About Thrombotic and Bleeding Complications in COVID-19?
Many people have suggested that COVID-19 represents a prothrombotic state that correlates with disease severity. However, this conclusion is based primarily on anecdotal and observational reports which to date have focused mainly on thrombotic risk to the exclusion of potential bleeding risk (T. Wang et al. 2020).
Lab Abnormalities:
Multiple case series have demonstrated an association between severe COVID-19 and abnormal coagulation markers. In a report of 183 patients with COVID-19, 71.4% of non-survivors met laboratory criteria for disseminated intravascular coagulation (DIC) compared to 0.6% of survivors (Tang et al. 2020). The clinical severity of SARS-CoV-2 infection, bleeding or thrombosis are not discussed. However, though DIC is associated with high mortality in COVID-19 in this report, it is still uncommon.
Spiezia et al. described the coagulation abnormalities in 22 consecutive patients admitted over 12 days to the Padova University Hospital ICU with COVID-19 associated respiratory failure. This group was compared to 44 healthy, matched controls. Here they found fibrinogen and D-dimer levels were significantly higher in COVID-19 patients than controls (p < 0.0001 in both comparisons) (Spiezia et al. 2020).
Clinical Thrombosis:
In a case series of 184 ICU patients with COVID-19 in the Netherlands, Klok et al. reported thrombotic complications in 31 patients (16.8%) (Klok et al. 2020). Lodigiani et al. examined thromboembolic complications in 48 consecutive patients admitted to the ICU with COVID-19, and 8 patients (16.7%) had at least one thromboembolic event (Lodigiani et al. 2020). Finally, in a recently published cohort study of 66 mechanically ventilated patients with COVID-19, 11 developed a thromboembolic event, excluding dialysis circuit thrombosis (16.7%) (Ziehr et al. 2020).
Llitjos et al. screened 26 consecutive patients admitted to two French ICUs with COVID-19-associated respiratory failure (81% with ARDS) for DVT using doppler ultrasound. This small study suggests a relatively high incidence of DVT (50%), but the way in which findings are reported make it difficult to interpret further. This high incidence may be related to systematic screening (and identify DVTs which would have otherwise been clinically silent) and the severity of illness in the cohort.
Ciu et al. reviewed records of 81 consecutive patients with COVID-19 admitted to the ICU and identified VTE events in 20 patients (25%) (Cui et al. 2020).
A separate French series reported a 20.6% incidence of PE in 107 patients admitted to ICU, representing an absolute increased risk of 14.4% (95% CI 6.1 to 22.8%) compared to a matched influenza cohort with similar illness severity score (Poissy et al. 2020). The median time from ICU admission to PE diagnosis was 6 days, and nearly all patients with PE had been on standard thromboprophylaxis with UFH or LMWH (others on full dose anticoagulation already). Similarly this group found an association between elevated D-dimer, plasma factor VIII activity, and von Willebrand factor levels with greater PE risk.
In 393 patients with COVID-19 in NYC, the rate of VTE in intubated patients was 7.7% (compared to 1.1% in non-intubated patients) (Goyal et al. 2020). This case series does not specify how many patients were screened for VTE, the type of VTE, or how VTE were diagnosed.
In contrast to the above reports, Cattaneo et al. found a zero percent incidence of DVT among 388 COVID-19 patients admitted to the general medical floor and given standard prophylaxis with 40mg daily of enoxaparin (Cattaneo et al. 2020).
There are reports of strokes in COVID-19 patients – a case series in NEJM described clinical features of 5 non-critically ill patients less than 50 years old with COVID-19 admitted with large vessel ischemic stroke (Oxley et al. 2020). Importantly, none of the patients in this case series were critically ill. Indeed, two patients in this series had no symptoms that were consistent with SARS-CoV-2 infection and one has a personal history of stroke - raising the possibility that the SARS-CoV-2 diagnosis was incidental to the diagnosis of stroke. Though this small case series has garnered substantial attention, it is presently unclear whether COVID-19 confers an increased risk of stroke beyond that conferred by infection from other etiologies or critical illness alone.
Outcomes:
In a separate study in Wuhan, China, Tang et al. compared outcomes between 99 patients with severe COVID-19 who received heparin products for VTE prophylaxis for at least 7 days to 350 patients who did not receive heparin for at least 7 days (Tang, Bai, et al. 2020). In this non-randomized trial, 94/99 patients in the heparin group received prophylaxis with LMWH and 5/99 with UFH. The reasons for this are not described. Furthermore, the authors fail to address either the reason 350 patients admitted with severe COVID-19 did not receive VTE prophylaxis for the duration of their hospital stay, nor the duration of VTE prophylaxis exposure in the “non-heparin” group (non-heparin group being defined by lack of prophylaxis for at least seven days, in a hospitalization that may have been greater than seven days). Coagulation indices are provided, details about clinical course, thrombosis or bleeding complications were not included, outside of a brief mention in the discussion (“bleeding complications were unusual and commonly mild”). With these caveats in mind, the authors found that prophylaxis with heparin products was associated with lower mortality in patients with elevated sepsis-induced coagulopathy score ≥ 4 (40.0% vs. 64.2%, p = .029) and those with D-dimer > 6x the upper limit of normal (32.8% vs 52.4%, p = .017).
We note that bleeding complications from COVID-19 have not been reported.
Thromboprophylaxis Considerations
Prior to COVID-19, there have been strong professional society recommendations for providing pharmacological VTE prophylaxis in all acutely or critically ill patients at acceptable bleeding risk (Schünemann et al. 2018). Respiratory illnesses in general have been shown to be a risk factor for hospital-acquired VTE (Barba et al. 2010). However, thromboprophylaxis is not failsafe: in a cohort of all patients admitted to an ICU, VTE prophylaxis with heparin or enoxaparin failed in 12.5% of patients in whom a DVT or PE were diagnosed (Hanify et al. 2017).
In the French report demonstrating frequency of PE in patients with COVID-19, the high prevalence of obesity in the patient group led authors to hypothesize that a subtherapeutic prophylactic regimen can explain some of the increase in VTE (Poissy et al. 2020). Indeed, data in hospitalized obese patients have demonstrated a reduction in VTE events in high-dose thromboprophylaxis compared to standard-dose (with some data suggesting that high-dose heparin prophylaxis may have a higher bleeding risk than high-dose enoxaparin prophylaxis) (Mason et al. 2019; T.-F. Wang et al. 2014). Even in weight-adjusted protocols, a prospective cohort study demonstrated that in patients with a weight above 150 kg, only 60% reached the target level of anticoagulation (Stier et al. 2020).
As per standard-of-care, hospitalized patients with COVID-19 should receive thromboprophylaxis at a minimum, unless contraindicated. Clear protocols should be created for morbidly obese patients and patients with severe renal disease or fluctuating renal function, as these patients often need different doses of anticoagulation. There have been discussions of post-discharge extended thromboprophylaxis for patients with COVID-19, but again we lack data to support this notion and guidelines suggest against this practice (Schünemann et al. 2018).
Who Should Receive Therapeutic Anticoagulation and How Should Clinicians Evaluate for Potential Thrombotic Complications in COVID-19?
There are debates in the literature whether to treat critically ill patients with COVID-19 and/or patients with suggestion of a pro-thrombotic or high-risk phenotype (i.e. high D-dimer, abnormal other coagulation markers, etc) with standard prophylactic anticoagulation, intermediate-dose prophylaxis or even full-dose anticoagulation. Strategies proposed include therapeutic heparin, low-molecular weight heparin, and even tissue plasminogen activator (Croce 2020). While there is biologic plausibility, there are no randomized data to support these strategies. Recent expert opinion recommends prophylactic measures for DVT prevention in critically ill COVID-19 patients, even if there is evidence of coagulopathy, reserving systemic anticoagulation only for standard indications (Connors and Levy 2020).
On the other hand, in addition to the largely observational data discussed above, there is some historic evidence in favor of escalated anticoagulation. In critically ill patients with severe ARDS, those with H1N1 viral pneumonia had a 23.3 fold higher risk for PE and 17.9-fold increased risk for VTE than patients without H1N1 (Obi et al. 2019). While this study was not randomized, H1N1 cases followed a bimodal distribution, and patients in the early cohort were treated with thromboprophylaxis alone. However, due to high rates of VTE observed, the hospital protocol for the later cohort included empiric treatment dose anticoagulation for all H1N1 ARDS patients unless contraindication was present. H1N1 patients without empiric anticoagulation were 33 times more likely to have any VTE compared to those with empiric anticoagulation with no differences in bleeding complications. There was no observed benefit to empiric anticoagulation in ARDS patients in the absence of H1N1 infection (see Figure). Conclusions from this study are limited due to non-randomized design but are hypothesis generating.
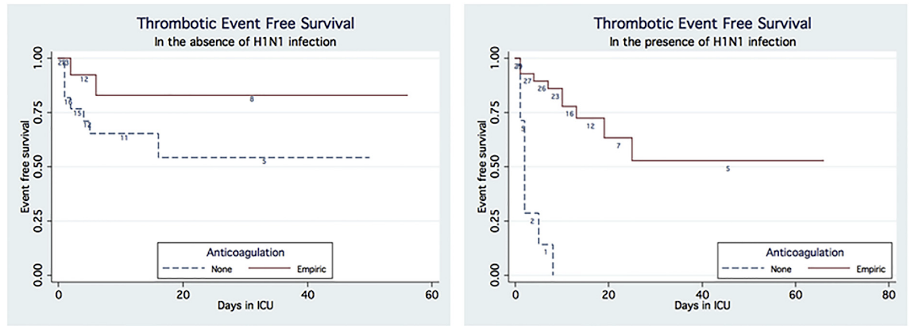
Figure 1
Kaplan-Meier plots of thrombotic events of patients with and without H1N1 infection who received and did not receive anticoagulation (Obi et al. 2019).
We currently await randomized clinical trial data to better inform whether high-risk COVID-19 patients should receive therapeutic anticoagulation, intermediate dose prophylaxis, or standard prophylaxis in absence of alternative indication for full anticoagulation. The risk of bleeding must also be weighed, particularly in this critically ill population; in data from China, patients at the highest risk of developing VTE were the same patients at highest risk of bleeding (T. Wang et al. 2020). In absence of evidence-based protocols to support full anticoagulation, the desire to aggressively screen patients for VTE must be balanced against the risk of viral transmission within the hospital due to increased diagnostic testing; elevated D-dimer alone does not warrant routine investigation for acute VTE. When possible, bedside studies may be preferred, acknowledging that lower limb venous ultrasound and echocardiography have variable and often limited sensitivity for PE, so negative ultrasonography should not reassure clinicians in context of high pre-test probability (Da Costa Rodrigues et al. 2016; Miniati et al. 2001; Bova et al. 2003).
Conclusions
Activation of the coagulation cascade and decreased fibrinolysis are observed in ARDS; however, to date, there are no data that demonstrate that escalation of anticoagulation beyond a prophylactic dose improves clinical outcomes. Observational and anecdotal data have generated hypotheses that COVID-19 confers a high risk of thrombosis, thus leading to the desire for aggressive anticoagulation in this population. At this time, we are also lacking data about bleeding risk in this population, despite the suggestion of increased rates of DIC. At the minimum, as standard of care, prophylactic dose anticoagulation should be administered to hospitalized patients with COVID-19 who do not have contraindication or clear indication for therapeutic anticoagulation. We await clinical trial data that will hopefully inform whether doses of anticoagulation beyond prophylactic dose modifies outcomes in patients admitted with COVID-19 with or without ARDS and mechanical ventilation.
References:
- Barba, Raquel, Antonio Zapatero, Juan E. Losa, Javier Marco, Susana Plaza, Jesús Canora, and Jose Manuel Casas. 2010. “Venous Thromboembolism in Acutely Ill Hospitalized Medical Patients.” Thrombosis Research 126 (4): 276–79.
- Bova, Carlo, Francesco Greco, Gianfranco Misuraca, Oscar Serafini, Francesco Crocco, Antonio Greco, and Alfonso Noto. 2003. “Diagnostic Utility of Echocardiography in Patients with Suspected Pulmonary Embolism.” The American Journal of Emergency Medicine 21 (3): 180–83.
- Camprubí-Rimblas, Marta, Neus Tantinyà, Josep Bringué, Raquel Guillamat-Prats, and Antonio Artigas. 2018. “Anticoagulant Therapy in Acute Respiratory Distress Syndrome.” Annals of Translational Medicine 6 (2): 36.
- Cattaneo, Marco, Elena M. Bertinato, Simone Birocchi, Carolina Brizio, Daniele Malavolta, Marco Manzoni, Gesualdo Muscarella, and Michela Orlandi. 2020. “Pulmonary Embolism or Pulmonary Thrombosis in COVID-19? Is the Recommendation to Use High-Dose Heparin for Thromboprophylaxis Justified?” Thrombosis and Haemostasis, April. https://doi.org/10.1055/s-0040-1712097.
- Connors, Jean Marie, and Jerrold H. Levy. 2020. “COVID-19 and Its Implications for Thrombosis and Anticoagulation.” Blood, April. https://doi.org/10.1182/blood.2020006000.
- Croce, Martin A. 2020. “Traumacare.” The Journal of Trauma and Acute Care Surgery 88 (1): 1–9.
- Cui, Songping, Shuo Chen, Xiunan Li, Shi Liu, and Feng Wang. 2020. “Prevalence of Venous Thromboembolism in Patients with Severe Novel Coronavirus Pneumonia.” Journal of Thrombosis and Haemostasis: JTH, April. https://doi.org/10.1111/jth.14830.
- Da Costa Rodrigues, Joao, S. Alzuphar, Christophe Combescure, G. Le Gal, and Arnaud Perrier. 2016. “Diagnostic Characteristics of Lower Limb Venous Compression Ultrasonography in Suspected Pulmonary Embolism: A Meta-Analysis.” Journal of Thrombosis and Haemostasis: JTH 14 (9): 1765–72.
- Dolhnikoff, Marisa, Amaro Nunes Duarte-Neto, Renata Aparecida de Almeida Monteiro, Luiz Fernando Ferraz da Silva, Ellen Pierre de Oliveira, Paulo Hilário Nascimento Saldiva, Thais Mauad, and Elnara Marcia Negri. 2020. “Pathological Evidence of Pulmonary Thrombotic Phenomena in Severe COVID-19.” Journal of Thrombosis and Haemostasis: JTH. https://onlinelibrary.wiley.com/doi/abs/10.1111/jth.14844.
- Goyal, Parag, Justin J. Choi, Laura C. Pinheiro, Edward J. Schenck, Ruijun Chen, Assem Jabri, Michael J. Satlin, et al. 2020. “Clinical Characteristics of Covid-19 in New York City.” The New England Journal of Medicine, April. https://doi.org/10.1056/NEJMc2010419.
- Greene, R., S. Lind, H. Jantsch, R. Wilson, K. Lynch, R. Jones, A. Carvalho, L. Reid, A. C. Waltman, and W. Zapol. 1987. “Pulmonary Vascular Obstruction in Severe ARDS: Angiographic Alterations after I.v. Fibrinolytic Therapy.” AJR. American Journal of Roentgenology 148 (3): 501–8.
- Hanify, Jennifer M., Lori H. Dupree, Donald W. Johnson, and Jason A. Ferreira. 2017. “Failure of Chemical Thromboprophylaxis in Critically Ill Medical and Surgical Patients with Sepsis.” Journal of Critical Care 37 (February): 206–10.
- Idell, Steven. 2003. “Coagulation, Fibrinolysis, and Fibrin Deposition in Acute Lung Injury.” Critical Care Medicine 31 (4 Suppl): S213–20.
- Klok, F. A., Kruip Mjha, N. J. M. van der Meer, M. S. Arbous, D. Gommers, K. M. Kant, F. H. J. Kaptein, et al. 2020. “Confirmation of the High Cumulative Incidence of Thrombotic Complications in Critically Ill ICU Patients with COVID-19: An Updated Analysis.” Thrombosis Research. https://doi.org/10.1016/j.thromres.2020.04.041.
- Lodigiani, Corrado, Giacomo Iapichino, Luca Carenzo, Maurizio Cecconi, Paola Ferrazzi, Tim Sebastian, Nils Kucher, et al. 2020. “Venous and Arterial Thromboembolic Complications in COVID-19 Patients Admitted to an Academic Hospital in Milan, Italy.” Thrombosis Research 191 (July): 9.
- Mason, S. Walker, Alexandra Barber, Emily Jones, Sheh-Li Chen, Stephan Moll, and Kalynn Northam. 2019. “Safety and Efficacy of High-Dose Unfractionated Heparin Versus High-Dose Enoxaparin for Venous Thromboembolism Prevention in Morbidly Obese Hospitalized Patients.” The American Journal of Medicine, December. https://doi.org/10.1016/j.amjmed.2019.12.003.
- Miniati, M., S. Monti, L. Pratali, G. Di Ricco, C. Marini, B. Formichi, R. Prediletto, et al. 2001. “Value of Transthoracic Echocardiography in the Diagnosis of Pulmonary Embolism: Results of a Prospective Study in Unselected Patients.” The American Journal of Medicine 110 (7): 528–35.
- Obi, Andrea T., Christopher J. Tignanelli, Benjamin N. Jacobs, Shipra Arya, Pauline K. Park, Thomas W. Wakefield, Peter K. Henke, and Lena M. Napolitano. 2019. “Empirical Systemic Anticoagulation Is Associated with Decreased Venous Thromboembolism in Critically Ill Influenza A H1N1 Acute Respiratory Distress Syndrome Patients.” Journal of Vascular Surgery: Venous and Lymphatic Disorders. https://doi.org/10.1016/j.jvsv.2018.08.010.
- Ospina-Tascón, Gustavo A., Diego F. Bautista, Humberto J. Madriñán, Juan D. Valencia, William F. Bermúdez, Edgardo Quiñones, Luis Eduardo Calderón-Tapia, Glenn Hernandez, Alejandro Bruhn, and Daniel De Backer. 2020. “Microcirculatory Dysfunction and Dead-Space Ventilation in Early ARDS: A Hypothesis-Generating Observational Study.” Annals of Intensive Care 10 (1): 35.
- Oxley, Thomas J., J. Mocco, Shahram Majidi, Christopher P. Kellner, Hazem Shoirah, I. Paul Singh, Reade A. De Leacy, et al. 2020. “Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young.” New England Journal of Medicine. https://doi.org/10.1056/nejmc2009787.
- Poissy, Julien, Julien Goutay, Morgan Caplan, Erika Parmentier, Thibault Duburcq, Fanny Lassalle, Emmanuelle Jeanpierre, et al. 2020. “Pulmonary Embolism in COVID-19 Patients: Awareness of an Increased Prevalence.” Circulation, April. https://doi.org/10.1161/CIRCULATIONAHA.120.047430.
- Rice, Todd W., Lewis Rubinson, Timothy M. Uyeki, Frances L. Vaughn, Benjamin B. John, Russell R. Miller 3rd, Elizabeth Higgs, et al. 2012. “Critical Illness from 2009 Pandemic Influenza A Virus and Bacterial Coinfection in the United States.” Critical Care Medicine 40 (5): 1487–98.
- Schünemann, Holger J., Mary Cushman, Allison E. Burnett, Susan R. Kahn, Jan Beyer-Westendorf, Frederick A. Spencer, Suely M. Rezende, et al. 2018. “American Society of Hematology 2018 Guidelines for Management of Venous Thromboembolism: Prophylaxis for Hospitalized and Nonhospitalized Medical Patients.” Blood Advances. https://doi.org/10.1182/bloodadvances.2018022954.
- Snow, R. L., P. Davies, H. Pontoppidan, W. M. Zapol, and L. Reid. 1982. “Pulmonary Vascular Remodeling in Adult Respiratory Distress Syndrome.” The American Review of Respiratory Disease 126 (5): 887–92.
- Spiezia, Luca, Annalisa Boscolo, Francesco Poletto, Lorenzo Cerruti, Ivo Tiberio, Elena Campello, Paolo Navalesi, and Paolo Simioni. 2020. “COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure.” Thrombosis and Haemostasis, April. https://doi.org/10.1055/s-0040-1710018.
- Stier, Christine, Ann-Cathrin Koschker, Raphael Stier, Alexander Sosnierz, and Sonja Chiappetta. 2020. “Are We Missing Treatment Standards for Thromboprophylaxis of the Obese and Super-Obese Patient Population? A Prospective Systematic Cohort Study.” Obesity Surgery 30 (5): 1704–11.
- Tang, Ning, Huan Bai, Xing Chen, Jiale Gong, Dengju Li, and Ziyong Sun. 2020. “Anticoagulant Treatment Is Associated with Decreased Mortality in Severe Coronavirus Disease 2019 Patients with Coagulopathy.” Journal of Thrombosis and Haemostasis. https://doi.org/10.1111/jth.14817.
- Tang, Ning, Dengju Li, Xiong Wang, and Ziyong Sun. 2020. “Abnormal Coagulation Parameters Are Associated with Poor Prognosis in Patients with Novel Coronavirus Pneumonia.” Journal of Thrombosis and Haemostasis: JTH 18 (4): 844–47.
- Wang, Tao, Ruchong Chen, Chunli Liu, Wenhua Liang, Weijie Guan, Ruidi Tang, Chunli Tang, Nuofu Zhang, Nanshan Zhong, and Shiyue Li. 2020. “Attention Should Be Paid to Venous Thromboembolism Prophylaxis in the Management of COVID-19.” The Lancet. Haematology, April. https://doi.org/10.1016/S2352-3026(20)30109-5.
- Wang, Tzu-Fei, Paul E. Milligan, Catherine A. Wong, Eli N. Deal, Mark S. Thoelke, and Brian F. Gage. 2014. “Efficacy and Safety of High-Dose Thromboprophylaxis in Morbidly Obese Inpatients.” Thrombosis and Haemostasis 111 (1): 88–93.
- Ziehr, David R., Jehan Alladina, Camille R. Petri, Jason H. Maley, Ari Moskowitz, Benjamin D. Medoff, Kathryn A. Hibbert, B. Taylor Thompson, and C. Corey Hardin. 2020. “Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study.” American Journal of Respiratory and Critical Care Medicine, April. https://doi.org/10.1164/rccm.202004-1163LE.
View all COVID-19 updates
Learn more about research in the Division of Pulmonary and Critical Care Medicine