New Data on Protective Immunity After COVID-19
The FLARE Four
- The world is eager for an “immunity test” for SARS-CoV-2, but the level and duration of protective immunity after recovery from COVID-19 remain to be defined
- Protective immunity depends on both production of antibodies by B cells and development of virus-specific T cell responses
- A new paper suggests not only that SARS-CoV-2-specific antibodies are detected in convalescent subjects, but that, in most of these individuals, antibodies are neutralizing and there is also evidence of a virus-specific T cell response
- While more work is needed, these results continue to provide assurance that natural infection will confer protective immunity, and that future vaccination may be effective
Many people are saying...once you've had COVID-19, you can't get it again.
Subscribe to the latest updates from FLARE Advances in Motion
Introduction
As discussed in a prior FLARE (April 24th), data from other coronaviruses and a small non-peer reviewed pre-print study using a rhesus macaque model of SARS-CoV-2 infection suggest that humans should possess protective immunity to SARS-CoV-2 after infection (Bao et al., 2020). As immunoglobulin tests have become more widely available, several governments have proposed “immunity passports” or “risk-free certification” to allow individuals to travel freely or return to work, assuming presence of IgG/IgM correlates with protective immunity. In tonight’s FLARE, we review new data on this topic - the first extensive characterization of SARS-CoV-2-specific cellular and humoral immunity in recovered COVID-19 patients (Ni et al., 2020).
Humoral and Cellular Immunity: A Brief Re-Introduction
The initial host response to infection depends on cells of the innate immune system, such as macrophages and neutrophils. Subsequent to this, the antigen-specific T and B cells of the adaptive immune system are activated and differentiate in response to the pathogen. B cells are responsible for the production of antibodies to pathogen-specific antigens, including viral structural proteins such as the spike (S) protein in the case of SARS-CoV-2. T cells comprise various subtypes that include CD4+ T lymphocytes (T helper cells, or Th cells) and CD8+ T lymphocytes (cytotoxic T cells, or Tc cells). CD8+ T lymphocytes are so-called cytotoxic T cells because they directly kill cells expressing viral antigens, whereas CD4+ lymphocytes classically secrete various cytokines that “help” stimulate other immune cells (see Figure 1 below).
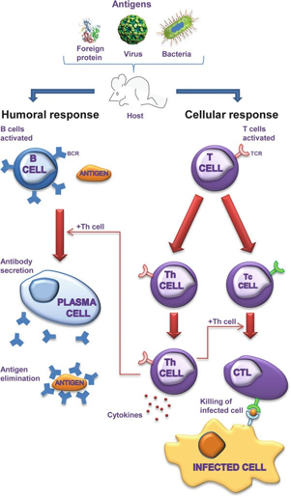
Figure 1
Schematic of adaptive and humoral immunity from (Bárcena and Blanco, 2013).
What Don’t We Know About SARS-COV-2 Serology?(Adapted From Developing a National Strategy for Serology (Antibody Testing) in the United States)
When considering host immune response to infection, there is an important distinction between what occurs in the host and what we are able to measure with currently available laboratory methods. Three specific questions are being asked about the serologic testing and response to COVID-19 disease that are not fully understood.
- Does the presence of antibodies correlate with protection? Currently it is unknown whether the antibodies evaluated in serologic tests actually neutralize the virus and if their presence protects from reinfection. At this time, there have been no human re-challenge experiments of convalescent patients
- How long does this protection last? If these antibodies do provide some protection against infection, it is unknown how long this protection lasts or if mutations in the virus will make reinfection possible. Immunity to SARS-CoV-2 may require an interplay between memory B and T cells, and the fluctuations in these populations over time are unknown
- Are serologic tests specific for SARS-CoV-2? Coronaviruses are widespread and some studies indicate that 100% of adults have some sort of antibody to a type of coronavirus (Gorse et al., 2010). We do not yet know if the serologic tests for SARS-CoV-2 related antibodies will cross-react (or interfere) with antibodies present from other coronavirus infections
What Did We Already Know About the Adaptive Immune Response in SARS-COV-2 Infection?
Several studies have provided some early evidence for adaptive immunity in SARS-CoV-2. Zhou and colleagues detected antibodies in 5 patients against multiple SARS-CoV-1 (also known as SARS-CoV) antigens that have substantial sequence similarity with the corresponding SARS-CoV-2 protein (Zhou et al., 2020). Antibody titers increased over the course of infection and IgG remained positive 20 days after disease onset. Furthermore, IgG-positive sera from these patients inhibited SARS-CoV-2 entry in vitro, suggesting a neutralizing property of these antibodies.
Wölfel and colleagues examined antibodies against the SARS-CoV-2 spike (S) protein, and detected seroconversion of IgG and IgM among 31 patients who had initially tested positive for SARS-CoV-2 infection via RT-PCR and had a mild course. Seroconversion occurred in 50% of patients by day 7, and 100% by day 14. They isolated no virus after day 7, and all patients showed detectable neutralizing antibodies (Wölfel et al., 2020). Of note, the group collected extensive viral load and serological information, and when comparing the results of simultaneous viral and serological testing, the authors described a gradual (not rapid) decline of viral load in the sputum after seroconversion.
In a non-peer reviewed pre-print article, Amanant and colleagues detected anti-S protein antibodies in three COVID-19 patients as early as 3 days after symptom onset (Amanat et al., 2020). Another case report showed increased antibody-secreting B-cells, follicular helper T cells, CD4+ T cells, CD8+ T cells, immunoglobulin M (IgM) and IgG antibodies that bound SARS-CoV-2 in blood of one patient before symptomatic recovery and for at least 7 days following full resolution of symptoms (Thevarajan et al., 2020).
What Does This New Study Add?
The study by Ni et al. attempts a more complete serological assessment of a small cohort of patients (Ni et al., 2020). This study endeavors to answer two fundamental questions: do COVID-19 patients develop SARS-CoV-2-specific antibodies, and do these antibodies have neutralizing activity against SARS-CoV-2? The authors evaluated blood samples from 8 recently discharged patients and 6 patients 2-weeks post-discharge after confirmed SARS-CoV-2 infection in Wuhan, China. All patients presented with mild symptoms and were confirmed positive by SARS-CoV-2 nucleic acid testing.
In order to detect SARS-CoV-2-specific antibodies, the investigators generated and purified the following recombinant SARS-CoV-2-derived proteins as antigens: the viral nucleocapsid protein (NP), receptor-binding domain of the S protein (S-RBD), and the main protease (MPRO) (see Figure 2 below). There was no significant MPRO antibody level, but NP- and S-RBD-specific IgM and IgG antibodies were both detected in sera of the 8 recently discharged patients and in the sera of the different 6 patients who were tested two weeks post-discharge. These antibodies were not detected in healthy controls. IgM titers were lower in the patients who were tested 2 weeks after discharge compared to those who were tested at discharge. In all, these results confirm that COVID-19 patients appear to generate IgM and IgG antibodies to SARS-CoV-2 proteins, including NP and S-RBD, and that infected patients may maintain IgG levels for at least 2 weeks after hospital discharge.
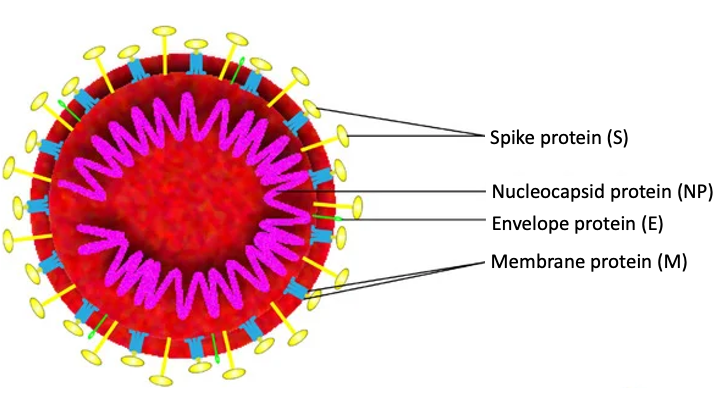
Figure 2
Image representing the overall structure of SARS-CoV-2 (Zhang et al., 2020).
What about neutralization? The authors used a neutralization assay to confirm a strong correlation between S-RBD antibody titer and its neutralizing activity. The well-recognized role of the S-protein in viral entry into cells may explain this finding (Hoffmann et al., 2020). Interestingly, NP antibody did not correlate with neutralizing activity. Thus, the COVID-19 patients at the time of hospital discharge had antibody-containing serum (and presumably can produce more antibodies) that should be able to neutralize SARS-CoV-2 infection. This result is consistent with a non-peer reviewed pre-print, which showed neutralizing antibody response to SARS-CoV-2 in a much larger cohort of patients who had recovered from COVID-19 (Wu et al., 2020).
Additionally, in an investigation that sets the report by Ni et al. apart from prior publications, they made an assessment of cellular immune responses to SARS-CoV-2. The study tested peripheral blood mononuclear cells from convalescent patients in response to recombinant SARS-CoV-2 antigens, and detected an increase in the number of IFN-γ-secreting T cells in recovered patients compared to healthy donors. This result suggests a SARS-CoV-2-specific T cell response. This is encouraging because antigen-specific T cell response may be even more durable than antigen-specific B cell response (see April 24th FLARE). Overall, the development of neutralizing antibodies correlated with the activation of IFN-γ-secreting T cells, which suggests an integrated humoral and cellular immune response.
What Are the Important Next Steps?
The study by Ni et al. provides encouraging, but not definitive, data that humans will develop protective immunity after COVID-19. However, similar to all of the prior COVID-19 immunology studies, the paper by Ni et al. had a very small cohort of patients, so larger studies across a variety of age ranges and severity of illness are needed. Furthermore, this study does not have data about changes in humoral and cellular immunity over time in the same patients. Lastly, studies will be needed to define the “immune correlates” of protective immunity. That is, what level of antibody or memory T cell pool size is needed to confer host protection? As noted in a prior FLARE, in both SARS and MERS, individuals who suffered more severe primary infection seemed to exhibit more long-lasting, antigen-specific immune memory (Memish et al., 2020; Tang et al., 2011; Zhao et al., 2017).
Conclusion
While this study provides the most comprehensive evidence that individuals who recover from COVID-19 will acquire protective immunity, the duration of such protection remains unknown. The conclusion of our April 24th FLARE holds: if SARS-CoV-2 establishes itself in the human population and protective immunity wanes over time, the development of an effective vaccine will be essential to protect both naïve individuals as well as survivors with waning immunity.
We also await a comprehensive serological assessment of patients with severe COVID-19. Could disease severity correlate with inadequate early humoral response? Or does increased disease severity actually lead to a more robust antibody response, perhaps increasing duration of immunity? As antibody tests become more widely used, especially given FDA’s emergency use authorization of an antibody test by Roche on May 5th, 2020, we anticipate answers to these important questions in the next few weeks.
References:
- Amanat, F., Stadlbauer, D., Strohmeier, S., Nguyen, T., Chromikova, V., McMahon, M., Jiang, K., Asthagiri-Arunkumar, G., Jurczyszak, D., Polanco, J., et al. (2020). A serological assay to detect SARS-CoV-2 seroconversion in humans (medRxiv).
- Bao, L., Deng, W., Gao, H., Xiao, C., Liu, J., Xue, J., Lv, Q., Liu, J., Yu, P., Xu, Y., et al. (2020). Lack of Reinfection in Rhesus Macaques Infected with SARS-CoV-2.
- Bárcena, J., and Blanco, E. (2013). Design of novel vaccines based on virus-like particles or chimeric virions. Subcell. Biochem. 68, 631–665.
- Gorse, G.J., Patel, G.B., Vitale, J.N., and O’Connor, T.Z. (2010). Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clin. Vaccine Immunol. 17, 1875–1880.
- Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., Schiergens, T.S., Herrler, G., Wu, N.-H., Nitsche, A., et al. (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell.
- Memish, Z.A., Perlman, S., Van Kerkhove, M.D., and Zumla, A. (2020). Middle East respiratory syndrome. Lancet 395, 1063–1077.
- Ni, L., Ye, F., Cheng, M.-L., Feng, Y., Deng, Y.-Q., Zhao, H., Wei, P., Ge, J., Gou, M., Li, X., et al. (2020). Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity 0.
- Quinti, I., Lougaris, V., Milito, C., Cinetto, F., Pecoraro, A., Mezzaroma, I., Mastroianni, C.M., Turriziani, O., Bondioni, M.P., Filippini, M., et al. (2020). A possible role for B cells in COVID-19?: Lesson from patients with Agammaglobulinemia. J. Allergy Clin. Immunol.
- Tang, F., Quan, Y., Xin, Z.-T., Wrammert, J., Ma, M.-J., Lv, H., Wang, T.-B., Yang, H., Richardus, J.H., Liu, W., et al. (2011). Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J. Immunol. 186, 7264–7268.
- Thevarajan, I., Nguyen, T.H.O., Koutsakos, M., Druce, J., Caly, L., van de Sandt, C.E., Jia, X., Nicholson, S., Catton, M., Cowie, B., et al. (2020). Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 26, 453–455.
- Wölfel, R., Corman, V.M., Guggemos, W., Seilmaier, M., Zange, S., Müller, M.A., Niemeyer, D., Jones, T.C., Vollmar, P., Rothe, C., et al. (2020). Virological assessment of hospitalized patients with COVID-2019. Nature.
- Wu, F., Wang, A., Liu, M., Wang, Q., Chen, J., Xia, S., Ling, Y., Zhang, Y., Xun, J., Lu, L., et al. (2020). Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications (medRxiv).
- Zhang, J., Zeng, H., Gu, J., Li, H., Zheng, L., and Zou, Q. (2020). Progress and Prospects on Vaccine Development against SARS-CoV-2. Vaccines (Basel) 8.
- Zhao, J., Alshukairi, A.N., Baharoon, S.A., Ahmed, W.A., Bokhari, A.A., Nehdi, A.M., Layqah, L.A., Alghamdi, M.G., Al Gethamy, M.M., Dada, A.M., et al. (2017). Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol 2.
- Zhou, P., Yang, X.-L., Wang, X.-G., Hu, B., Zhang, L., Zhang, W., Si, H.-R., Zhu, Y., Li, B., Huang, C.-L., et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273.
Learn more about research in the Division of Pulmonary and Critical Care Medicine
View all all COVID-19 updates