Artificial Intelligence Improves Treatment Monitoring in Patients with Glioma
In This Article
- Assessment of response to treatment in glioma is currently based on manual measurements of tumor burden
- Such measurements are time-consuming and variable, limiting both their utility and their reliability
- The Department of Radiology at Massachusetts General Hospital is now working to implement an artificial intelligence algorithm developed by Mass General researchers that will advance treatment monitoring by enabling automatic tumor segmentation
An estimated 78,000 primary brain and other central nervous system (CNS) cancers will be diagnosed in the U.S. this year. Researchers are actively developing new therapies for glioma, the most common type of primary brain tumor. Challenges remain, however, in assessing whether patients are responding to treatment.
Subscribe to the latest updates from Radiology Advances in Motion
To address these challenges, researchers at the Athinoula A. Martinos Center for Biomedical Imaging in the Department of Radiology at Massachusetts General Hospital have devised a deep learning algorithm. The algorithm automatically measures tumor volume based on MRI images, providing more rapid and more consistent treatment monitoring for patients. The researchers are now working to integrate the algorithm into the neuroradiology and neuro-oncology clinical workflows at Mass General.
Using Deep Learning, Developers Address Limitations in Current Gold Standard Measurements of Treatment Response
In current practice, assessing a glioma patient’s response to treatment is based on manual measurements of tumor burden guided by Response Assessment in Neuro-Oncology (RANO) criteria. Using these criteria, clinicians can identify the response as “complete response,” “partial response,” “stable disease” or “progression,” based on specific imaging and clinical features. The RANO-based method offers a beneficial approach to treatment monitoring but is time-intensive and can vary depending on who performs the measurement. Also, to facilitate ease of use for the clinicians doing the reading, the approach relies on bi-directional diameter measures. Such measures, however, are unlikely to provide an accurate representation of the size of the tumor since gliomas are often irregular in shape. Volumetric measures would be more valuable for treatment monitoring purposes, but such measures would be impracticable for manual assessments of tumor burden because of the time and effort required.
The Martinos Center researchers therefore developed an algorithm for automatic measurement of tumor volumes as well as bi-directional measurement (an approach they call AutoRANO). This work grew out of recent advances in the field of deep learning, a type of artificial intelligence capable of performing complex tasks by using layers to learn progressively higher-level features from an image. The researchers built the algorithm using DeepNeuro, an open-source deep learning software package for neuroimaging developed by the Quantitative Translational Imaging in Medicine (QTIM) laboratory at the Martinos Center. In doing so, they worked closely with physicians from both Mass General and Brigham and Women’s Hospital to fully optimize it for clinical use.
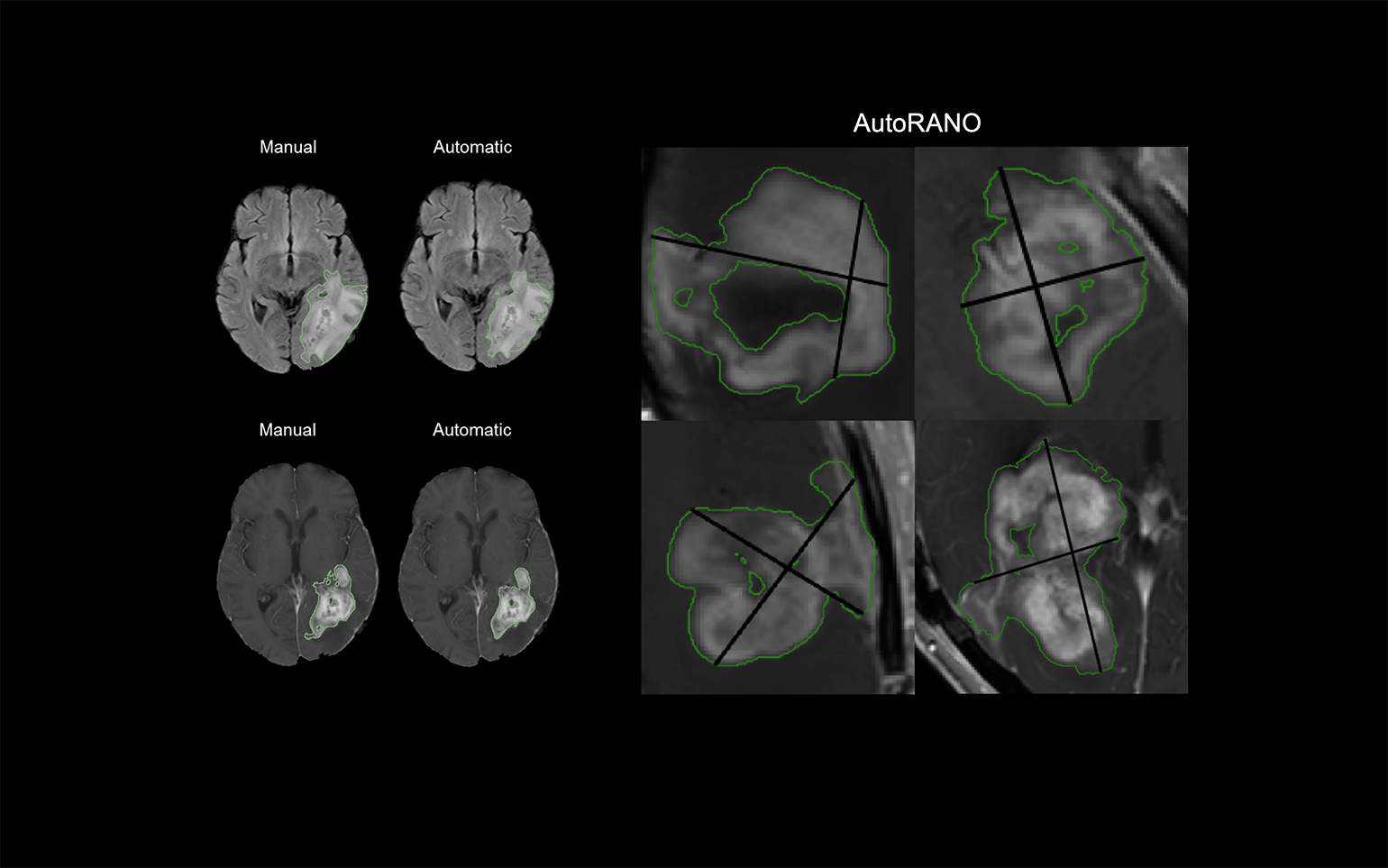
Figure 1
The Department of Radiology at Massachusetts General Hospital will soon have improved treatment monitoring in patients with glioma thanks to an artificial intelligence algorithm developed by Mass General researchers. Using an artificial intelligence technique called deep learning, the algorithm learns and then performs automatic tumor segmentation and determination of tumor volumes based on Response Assessment in Neuro-Oncology (RANO) criteria, thus allowing faster and more accurate measurement of tumor burden
In a 2019 study reported in the journal Neuro-Oncology, the team validated the algorithm’s performance by comparing the automated measurements in two patient cohorts with manual measurements by experts. They first demonstrated the algorithm’s superiority in learning and performing brain extraction, a challenging preprocessing step often giving rise to difficulties downstream. Its strength in this area enabled robust tumor segmentation based on both fluid-attenuated inverse recovery (FLAIR) hyperintensity and contrast enhancement. The tool also performed well in automatically calculating RANO measurements from the contrast enhancement segmentations, showing high agreement with the manually generated tumor volumes. With these results and other findings, the study underscored the possible clinical utility of the algorithm in patients with glioma.
Clinical Workflows in Neuroradiology and Neuro-oncology to Benefit from Artificial Intelligence
The research team is now actively working with information technology engineers to add the algorithm into clinical workflows at Mass General for neuroradiology and neuro-oncology by the end of 2020. The goal is a tool with a user-friendly interface that seamlessly incorporates into the radiology picture archiving and communication system (PACS).
Once integrated into workflows, the algorithm has the potential to offer a host of benefits for physicians and patients. In addition to providing more reliable quantitative metrics to aid with clinical decision-making in treatment monitoring, it could also foster development of new treatments for patients with glioma within clinical trials. It could also work well with other automated tools currently under development in the QTIM Laboratory, including an algorithm that can predict particular mutations in patients with glioma using MRI data. These mutations are associated with longer survival and could help guide treatment decisions.
Visit the Athinoula A. Martinos Center for Biomedical Imaging
Learn more about the Department of Radiology at Mass General