PEEP, ARDS and COVID-19 Associated Respiratory Failure
The FLARE Four
- Respiratory failure in COVID-19 is ARDS and should be treated as such
- Standard of care in ARDS is low Vt ventilation, conservative fluid management, judicious PEEP, prone ventilation, typically provided in that order
- There is evidence that COVID-19 pts frequently have a biomarker profile consistent with hyperinflammatory ARDS (in particular, elevated IL-6). The treatment implications of a hyperinflammatory biomarker profile are, to date, uncertain
- Summary of major PEEP strategy trials: ALVEOLI - High PEEP vs. Low PEEP; EXPRESS - High PEEP vs. Moderate PEEP; and LOVS - Open lung approach vs. Low Vt
Today's FLARE email deals extensively with PEEP and the appropriate way to set it in ARDS in general and COVID-19 associated respiratory failure.
Subscribe to the latest updates from FLARE Advances in Motion
- Discussion of ARDS, PEEP strategy and ARDS subphenotypes in COVID-19 by Dr. Corey Hardin
- Summary of major PEEP strategy trials:
- ALVEOLI - High PEEP vs. Low PEEP
- EXPRESS - High PEEP vs. Moderate PEEP
- LOVS - Open lung approach vs. Low Vt
Is Respiratory Failure in the Setting of COVID-19 ARDS?
(Summary of Guan et. al, NEJM 2020. Lung Safe 2016; 315(8):788 and various informal case series)
The answer to this is almost certainly yes. In the largest published case series from China (Guan et. al. m NEJM) almost 90% of CT scans performed on hospital admission already had significant abnormalities and over 50% of those were bilateral. In other words, patients already met most of the Berlin definition ARDS on hospital admit. This is a point that Taylor Thompson has made repeatedly. Anyone who subsequently developed hypoxemia requiring any significant degree of supplemental O2 would then be considered to have ARDS by the formal definition. However, it is important to remember that ARDS has always been a disease with an extremely diverse presentation. We are seeing lots of reports from centers around the world that make the case that COVID-19 represents a special cohort because of patients presenting with higher P:F ratios, lower PEEP (8-12) and near normal compliance. Essentially a fairly mild presentation that has been characterized as ‘viral pneumonia only’ and not florid ARDS. I would agree that it is not severe ARDS, but that has always been only one end of a spectrum of disease. In the Lung-Safe study, which is a large observational cohort of ARDS cases world-wide patients overall had a mean P:F of 165 and mean PEEP of ~8. In that same cohort, mean Pplat was only 23. So we should avoid re-defining ARDS to mean just severe, low compliance ARDS. The point is not that these patients do not behave differently than the more severe cases, it’s just that they have always been in our trials and we should not discard what we know from those trials – instead these data are very applicable to the current situation. The good news is that these milder presentations were associated with a lower ICU and 28-day mortality in Lung Safe.
As the outbreak has progressed, we increasingly have access to both published and unpublished case series. Many of these circulate informally and amount to summations of the experience of individual providers. For example, a “note from an anonymous Seattle intensivist” was widely circulated over email in early March and more of these have come out as the outbreak spreads to additional cities and countries. It is important to keep in mind that such personal case series are highly likely to dominated by impressions from cases with high emotional valence and we should not over generalize. Nevertheless, a consensus does seem to be developing that respiratory failure from SARS-CoV2 infection alone is associated with a relatively mild pulmonary mechanical impairment that responds to moderate PEEP and prevention of further lung injury by means lung protective mechanical ventilation. This is mild to moderate ARDS. The more severe, consolidated, and low compliance patients may also have a degree of ventilator-induced lung injury (VILI) or self-induced lung injury (SILI), for example in patients exposed to NIPPV and/or HFNC. It is, however, the case that the group of patients with severe ARDS in historical trials has always been enriched with patients who have a degree of VILI/SILI.
What is the Standard Approach to ARDS That Might Be Applicable to COVID-19 Patients?
(With reference to ARMA, FACTT, LOVS, EXPRESS, ALVEOLI, ART (JAMA 318(14):1335) and PROSEVA (NEJM 2013; 368:2159-2168))
Since everything we are seeing about these patients indicates that they are similar to normal ARDS patients, if somewhat milder than might have been expected, we can have great confidence in applying the evidence-based supportive care approach that has been developed by ARDSnet/PETAL and others over the last 50 years of ARDS research. This is one reason why there is such a high degree of similarity between the various treatment guidelines for ICU care of COVID-19 patients that are now publicly available such our own, those published by the Brigham and Women’s Hospital and those published last week by the Society of Critical Care Medicine. All of the guidelines, including the MGH ones, amount to reiteration of standard evidenced-based critical care. The papers mentioned above constitute the core of the evidence base for the critical care of ARDS. All the available guidelines urge low tidal volume ventilation (as outlined in the classic ARMA trail), conservative fluid management (as outlined in the FACTT trial) , judicious use of PEEP in the event of poor mechanics or poor oxygenation and prone ventilation for those failing to respond (associated with a substantial mortality benefit in PROSEVA) and judicious use of PEEP in the event of poor mechanics or poor oxygenation.
There are two places where the Mass General guidelines diverge more significantly from those put out by the SCCM. These are in the use of HFNC and/or NIPPV and the use of high PEEP. The Mass General guidelines discourage the use of HFNC and/or NIPPV in COVID-19 patients and the SCCM guidelines suggest their use in order to forestall intubation. The SCCM guidelines suggest the use of high PEEP and/or recruitment maneuvers and the MGH guidelines de-emphasize recruitment maneuvers and high PEEP, while acknowledging that select patients may benefit. The issue of NIPPV and HFNC is a significant one and will be dealt with at length in a subsequent FLARE email. Tonight’s email deals extensively with the issue of PEEP and the appropriate way to set it in ARDS in general and COVID-19 associated respiratory failure in particular. We provide summaries of the three major, early trials of high PEEP approaches EXPRESS, ALVEOLI and LOVS. These three trials have in common that they showed no mortality benefit to high PEEP and/or recruitment strategies. This was surprising, because the physiologic rationale for PEEP and recruitment maneuvers is clear – in ARDS the critical opening pressure of some lung units is increased, leading to those units being under ventilated at normal airway pressures and, to the extent that they are still perfused, increased shunt and arterial hypoxemia. These units may, in fact, require quite elevated trans-pulmonary pressures. Fortunately, the lung pressure-volume is characterized by substantial hysteresis so that the pressure required to keep a lung unit open may be substantially less than that needed to open it. These observations seem to constitute a compelling rationale for a high-driving pressure recruitment maneuver followed by relatively high PEEP and this approach came to be known as the open lung approach. Despite the discouraging clinical results, subsequent meta-analysis suggested that there did exist a subset of patients who benefit from an open lung approach. This was the background to the ART trail published in 2017 which tested a formal recruitment maneuver followed by physiologically titrated PEEP based on the best tidal compliance. This trial generated great interest as in showed an increase in mortality in the treatment arm. Three randomized trials with no benefit and one that suggested harm constitute an impressive argument against an open lung approach. Nevertheless, there are problems with ART. If the contention was that only a subset of patients benefit from PEEP, it was somewhat unsatisfying that ART made no attempt to identify this subgroup. In fact, the difference in driving pressure between the two arms of ART was only 2cm H2O which indicates not much recruitment occurred, perhaps because the patients where not enriched with the subgroup purported to benefit. A fair summary of this literature is that the clinical evidence for high PEEP and recruitment for every patient is poor. However, it is still likely true that if driving pressure does not increase (or goes down) as PEEP increases then recruitment is occurring and this is likely to be beneficial. This summary forms the basis of the recommendations in our guidelines for use of the ARDSnet low PEEP table if patients are not meeting targets on initial settings and for careful individual PEEP titration if expert personal are available (to monitor driving pressure during the titration and determine if patients are truly recruitable).
What is the Evidence for a Hyperinflammatory Subphenotype in COVID-19 Associated Respiratory Failure?
(Mehta et. al. Lancet, March 13 2020, Famous et. al. Calfee et. al. Shakoory et. al. CCM 2016 44(2):275-281)
Last night, we reviewed two papers having to do with efforts to define sub-phenotypes in ARDS. The discussion above on the clinical presentation of ARDS in COVID-19 and on possible differential response to PEEP emphasize the heterogeneity of the disease. Many efforts have been undertaken to try to reduce heterogeneity by defining biomarker or clinical profiles that may differentially respond to treatment. A promising such endotype is so-called hyperinflammatory ARDS characterized by, among other things, elevated IL-6, reviewed last night. One intriguing treatment implication, also reviewed last night, is the suggestion that hyperinflammatory ARDS may be benefit from statins despite unselected ARDS patients showing no such benefit. There are some data to suggest that COVID-19 patients may fit into this category given their elevated inflammatory markers. For this reason, statins are suggested in our guidelines.
COVID-19 patients also show elevated IL-6 as indicated in Yuan et. al. In addition, it has been suggested that elevated levels of other inflammatory markers such as ferritin and CRP indicate that there exists a sub-type of COVID-19 that is characterized by a cytokine release syndrome similar to secondary haemophagocytic lymphohistiocytosis (sHLH)sHLH is being studied in sepsis, for example in the ongoing PROVIDE trial. In particular, since various strategies to reduce IL-1 signaling are effective treatments for the related syndrome of macrophage activation syndrome, there is interest in the use of similar strategies in sepsis and the purported sHLH associated wth COVID-19. HLH is typically diagnosed by a so-called Hscore with based on dramatic elevations in ferritin, organomegaly, leukopenias, fever etc. An Hscore of 169 is associated with a93% sensitivity and 86% specificity for diagnosis of sHLH. A phase III trial of IL-1 receptor blockade failed to show benefit in undifferentiated sepsis , but a post-hoc reanalysis suggested benefit in the subset of patients thought to have features of the macrophage activation syndrome defined as hepatobillary dysfunction and DIC. The story here is remarkably similar to the time course of other critical care research approaches – including PEEP – with only ambivalent signals of success. Moreover, the levels of elevated cytokines in COVID-19 are not in line with frank sHLH. Even the patients with the most elevated IL-6 levels reach levels only in the 200 ng/ml range and are thus more typical of usual hyperinflammatory ARDS than true sHLH. Reported ferritin levels are only in the 1000-2000 range, which is not high enough to count in the Hscore system at all. For this reason, our guidelines reflect some optimism about statins but recommend additional immunomodulation only as part of a clinical trial.
Where We've Been: ALVEOLI, EXPRESS and LOVS
Review of ALVEOLI, Courtesy of Dr. Anica Law
(Brower RG, Lanken PN, Macintyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327-36)
Background: High PEEP vs. low PEEP: high PEEP may improve O2, reduce VILI, but can worsen circulatory depression or overdistention
Methods: n = 549 ALI/ARDS pts (P:F ratio<300 + bilateral infiltrates, enrolled within 36 hrs of onset), randomized to low/high PEEP on MV (on 6cc/kg Vt, and Pplat <30cm), according to tables of PEEP/FiO2, below:
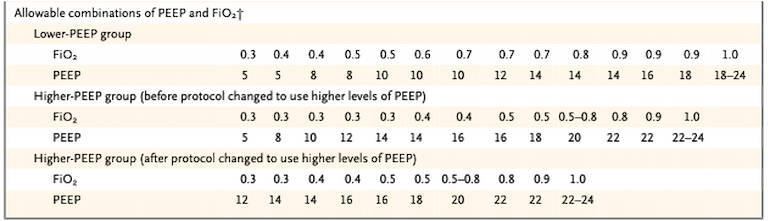
Table 1
*NOTE: 171 pts were enrolled initially and split between low vs. high PEEP groups, but then it was found that the difference in mean PEEP between the high/low PEEP groups was too small; the remaining 378 patients were randomized between a low and a new higher PEEP protocol.
Results:
- LOW PEEP group: Mean PEEP 8.3+/- 3.2cm H2O
- HIGH PEEP group: Mean PEEP 13.2 +/-3.5 cmH2O
Primary Outcome (Low vs. High), in-hospital mortality: 24.9% vs. 27.5% (p = 0.48, 95% CI for difference -10% to +4.7%).
Secondary Outcome, # of days with unassisted breathing out of 28 days (Low vs. High): 14.5+/-10.4 vs. 13.8+/-10.6 (p = 0.50).
Conclusion: Clinical outcomes are similar between high/low PEEP groups.
ALVEOLI was Followed By Two Trials, EXPRESS and LOVS, Studying the Impact of Alveolar Recruitment via Recruitment Maneuvers
Review of EXPRESS, courtesy of Dr. Anica Law
(Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655)
Question: Is a strategy that tries to increase recruitment via the highest possible PEEP (up to maximal plateau pressure) better than a moderate PEEP level (both with low tidal volumes)? (i.e. is it more important to increase alveolar recruitment or to minimize alveolar distention?)
Methods: n = 767 ALI patients at 37 centers in France (2002-2005); randomized to receive either PEEP up to plateau pressure of 28-30 cm H2O (“increase recruitment strategy”) or PEEP 5-9cm H2O (“minimal distention strategy”)
Results:
- LOW PEEP/minimal distention group: Mean PEEP
- HIGH PEEP/alveolar recruitment group: Mean PEEP
Primary outcome:
28 d. mortality (low PEEP vs. high PEEP): 31.2% vs. 27.8% (p = 0.31; RR 1.12 95% CI: 1.12, 0.90-1.40)
Secondary outcomes:
- 60d. hospital mortality: 39.0% vs. 35.4% (p = 0.30; RR 1.10, 95% CI 0.92-1.32)
- Vent free days: 3 (IQR 0-17) vs 7 (IQR 0-19), (p = 0.04)
- Organ-failure-free days at 27 days: 2 (IQR 0-16) vs. 6 (IQR 0-18) (p = 0.04)
Conclusions:
Compared to a moderate PEEP strategy, using a strategy to increase recruitment by maximizing PEEP up to an acceptable plateau pressure did NOT reduce mortality, but it did increase # of vent-free days and organ-failure-free days.
*Other noted side effects of high recruitment strategy: improved lung function (compliance, oxygenation, less adjunctive therapies), and more fluids required to support hemodynamics. No difference in PTX, vasopressor therapy, extubation failure.
Review of LOVS, Courtesy of Dr. Krishna Reddy
(Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645)
Objective: “To compare an established low-tidal-volume ventilation strategy with an experimental strategy based on the original ‘open-lung approach,’ combining low tidal volume, lung recruitment maneuvers, and high positive-end–expiratory pressure.”
Design/Setting: RCT, years 2000-2006, 30 ICUs in Canada, Australia, and Saudi Arabia.
Patients: 983 patients with acute lung injury/ARDS and P:F <250.
Exclusion criteria: Inability to wean from experimental strategies such as nitric oxide, severe chronic respiratory disease, >48 hours of eligibility, and a few others.
Experimental strategy: Tidal volume target 6 mL/kg, Pplat<40, recruitment maneuvers, higher PEEP levels (“lung open ventilation strategy”). Patients started with a recruitment maneuver, which included a 40-second breath-hold at 40 cm H2O airway pressure, on FIO2 of 1.0. An additional recruitment maneuver followed each disconnect from the ventilator, up to 4 times daily.
Control strategy: Tidal volume target 6 mL/kg, Pplat<30, conventional PEEP levels. Recruitment maneuvers were not permitted.
Primary outcome: All-cause hospital mortality.
Results: Mean PEEP was ~15 in experimental group and ~10 in control group during the first 72h (P<0.001). All-cause hospital mortality occurred in ~36% in experimental group and ~40% in control group (RR 0.90, 95% CI 0.77-1.05, P=0.19). There was no significant difference in barotrauma rates. The experimental group had significantly lower rates of prespecified secondary outcomes, including refractory hypoxemia, death with refractory hypoxemia, and use of rescue therapies; the point estimate of the risk ratio for each of these was ~0.5-0.6.
Conclusions: The study did not demonstrate that the “lung open ventilation strategy” results in significantly lower all-cause mortality compared with the established approach at the time. However, the “lung open ventilation strategy” improved secondary outcomes related to hypoxemia and rescue therapies, and there was no significant difference in barotrauma.
The takeaway message: Higher PEEP and recruitment maneuvers do not reduce all-cause mortality in ALI/ARDS, but they appear to reduce the risk of refractory hypoxemia and need for rescue therapies, and they do not appear to cause significant harm for most patients.
Implications for clinical care: There may be a subgroup of patients who benefit from higher PEEP (lung recruitment in those with more edema and collapse of dependent regions) and another subgroup who do not benefit or are harmed by higher PEEP (overdistention). Thus, in the absence of barotrauma, it is reasonable to try higher PEEP and recruitment maneuvers while monitoring Pplat, airway driving pressure (Pplat – PEEP), O2 saturation, and hemodynamics. Note that few patients in this study were proned.
Strengths of the study: Good adherence to protocols; complete follow-up; multicountry.
Limitations of the study: Unable to differentiate between effects of higher PEEP, higher Pplat, or recruitment maneuvers; some baseline differences between groups.
Details of interest:
- Among the motivations for a “lung open ventilation strategy”: trying to prevent atelectrauma (repetitive alveolar collapse with shearing), and potential oxygen toxicity from high FIO2
- In this study, the most common causes of lung injury were sepsis (47%), pneumonia (45%), and gastric aspiration (19%)
- ~44% of patients in both groups were treated with neuromuscular blockade
- The protocol adherence appeared to have been generally successful: the experimental group had significantly higher PEEP, higher Pplat, lower FIO2, and higher P:F ratios on days 1, 3, and 7, compared to the control group
- Patients in the experimental group were, on average, 2.4 years younger than those in the control group
- When adjusted for some baseline variables, including age and APACHE II score, the RR of all-cause hospital mortality in the experimental group was 0.97 (95% CI 0.84-1.12, P=0.74) compared to the control group
- Rescue therapies included inhaled nitric oxide, prone ventilation, high-frequency oscillation, high-frequency jet ventilation, and ECMO
- In the experimental group, 22% of patients developed a complication associated with a recruitment maneuver. These included hypotension, hypoxemia, and arrhythmia
References
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China [published online ahead of print, 2020 Feb 28]. N Engl J Med. 2020;10.1056/NEJMoa2002032.
- Bellani G, Laffey JG, Pham T, Fan E; LUNG SAFE Investigators and the ESICM Trials Group. The LUNG SAFE study: a presentation of the prevalence of ARDS according to the Berlin Definition. Crit Care. 2016;20(1):268.
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575.
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168.
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression [published online ahead of print, 2020 Mar 16]. Lancet. 2020.
- Famous KR, Delucchi K, Ware LB, et al. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy [published correction appears in Am J Respir Crit Care Med. 2018 Dec 15;198(12):1590.
- Calfee CS, Delucchi KL, Sinha P et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9):691-698.
- Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med. 2016;44(2):275–281.
- Brower RG, Lanken PN, Macintyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327-36.
- Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645.
- Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655.
- Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645.
- Cavalcanti AB, Suzumura ÉA, Laranjeira LN, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA. 2017;318(14):1335-1345.
View all COVID-19 updates
Learn about research in the Division of Pulmonary and Critical Care Medicine