Procalcitonin and SARS-CoV-2
The FLARE Four
- Procalcitonin level is frequently elevated by bacterial infections, but should not be the sole criterion for administering antibiotics
- Procalcitonin may be helpful in identifying bacterial co-infection in viral pneumonia
- Severity of SARS-CoV-2 infection is correlated with higher levels of procalcitonin. It is unclear if this is related to bacterial co-infection or severity of the viral infection itself
- As in other conditions, procalcitonin should be interpreted in the broader clinical context of COVID-19 associated respiratory failure. Elevated levels may suggest severe disease, bacterial co-infection, or both
Subscribe to the latest updates from FLARE Advances in Motion
Many people are wondering...what does an elevated procalcitonin level mean in SARS-CoV-2 infection?
What is Procalcitonin?
Procalcitonin (PCT) is a peptide synthesized by thyroid neuroendocrine cells (C cells) which normally undergoes proteolytic cleavage to yield the calcium-regulating hormone calcitonin. In healthy subjects, serum procalcitonin levels are undetectable by traditional assays (Maruna et al., 2000) as PCT is not released into the blood until it is cleaved into its mature form. In the presence of bacterial endotoxin or certain cytokines, however, procalcitonin synthesis is greatly upregulated in multiple tissues (Linscheid et al., 2003; Müller et al., 2001), with levels rising within hours and peaking over 1-2 days (Meisner, 2014, Linscheid et al., 2003; Müller et al., 2001).
Why Might Procalcitonin Be Helpful in Distinguishing Bacterial Infection from Viral Infection?
Bacterial antigens stimulate the release of inflammatory mediators such as TNFα, IL-1β, and IL-6 which, in turn, promote procalcitonin transcription. In contrast, during viral infection, T helper cells produce IFNγ, which is believed to inhibit procalcitonin production (Linscheid et al., 2003). For this reason, procalcitonin has been proposed as a serum biomarker to distinguish between bacterial and viral infections. However, it is important to note that procalcitonin may also be elevated in non-infectious inflammatory states such as burns, trauma, and paraneoplastic syndromes as well as after therapy with immune stimulating medications (Samsudin and Vasikaran 2017).
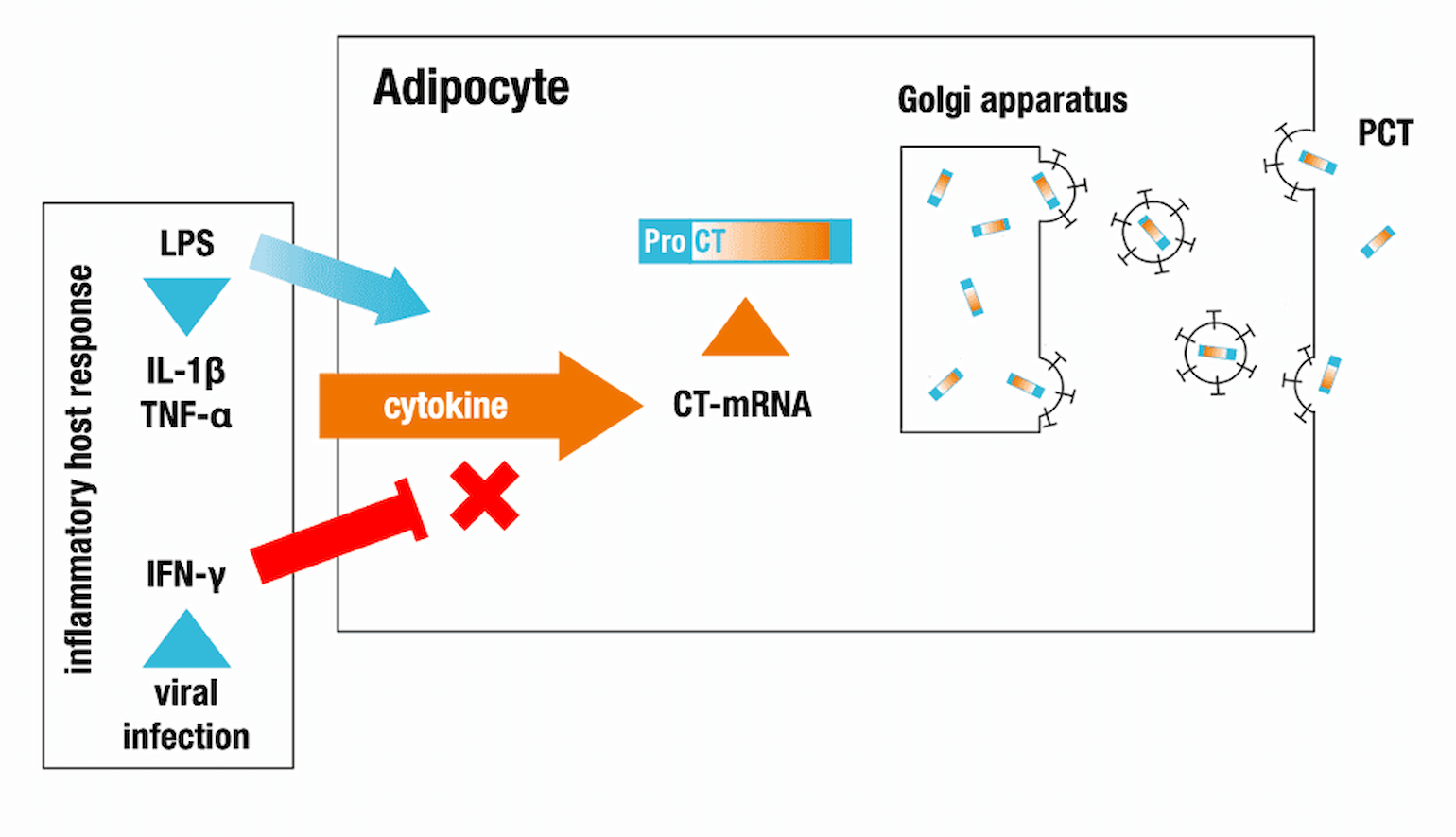
Figure 1
Proposed mechanism of procalcitonin (PCT) release. From Thermo-Fisher.
How Does Procalcitonin Assist Us in Clinical Practice?
A Cochrane review of 32 RCTs evaluated the initiation or discontinuation of antibiotics for acute respiratory infections (ranging from CAP to the “common cold”) found that procalcitonin-guided therapy decreased mortality (8.6% vs 10%) and decreased antibiotic exposure (5.7 days vs 8.1 days) with no difference in reported treatment failure (23.0% vs 24.9%) (Schuetz et al., 2017). Based on this report, some institutions use procalcitonin to evaluate patients with respiratory complaints.
Multiple studies evaluated procalcitonin levels in patients with viral respiratory infections who are susceptible to co-infection with bacteria. In many H1N1 studies, PCT level appears useful:
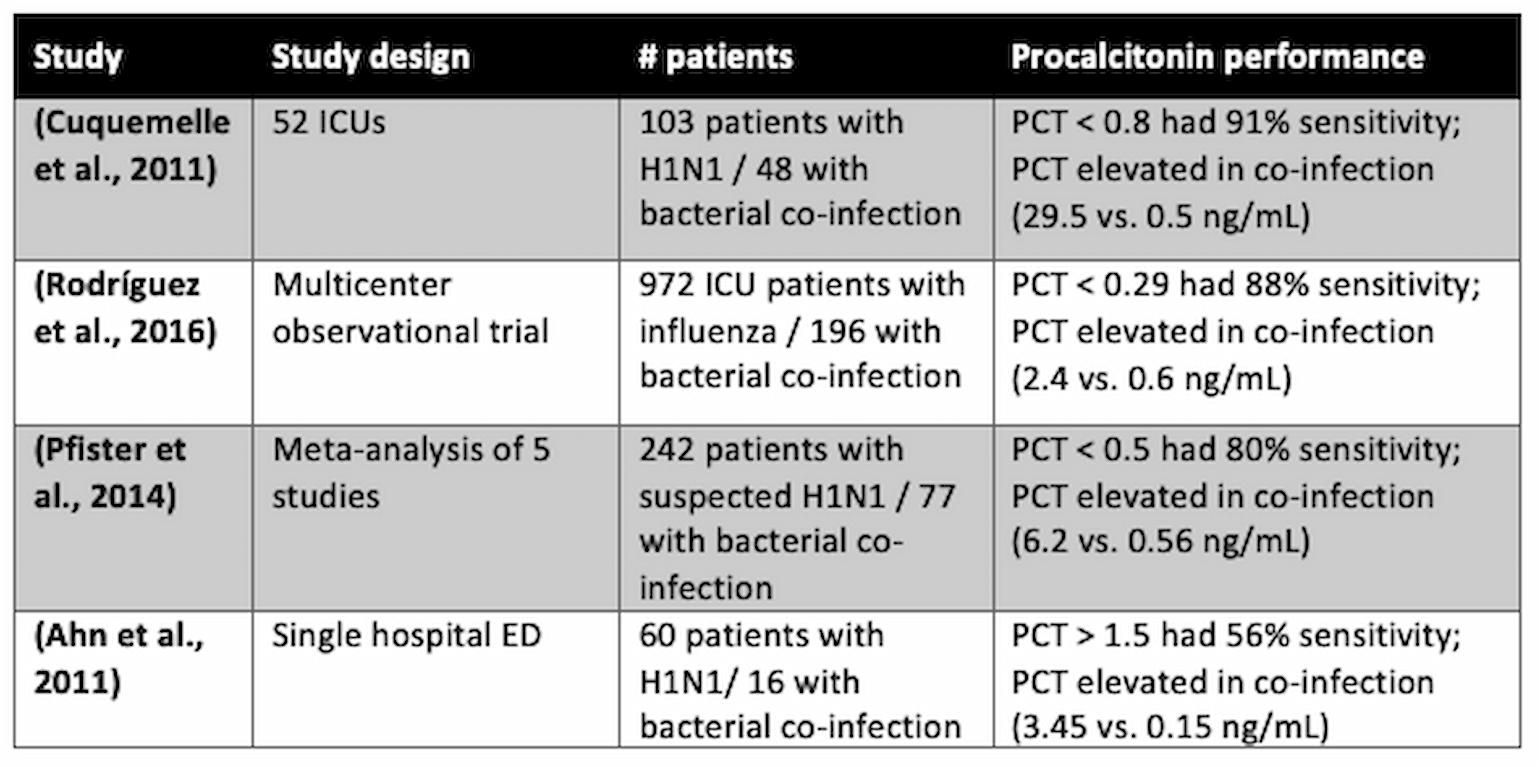
Table 1
Abbreviations: PCT = procalcitonin.
Furthermore, in a study 27 patients with respiratory failure from human metapneumovirus requiring ICU admission, 7 patients had S. pneumoniae co-infection. Median PCT level was 18 ng/mL in patients with a bacterial co-infection compared to 0.5 in all others (Vidaur et al., 2019).
Not all of the data has been as promising. A study of 38 patients with H1N1 pneumonia identified 8 patients with a bacterial co-infection, all with PCT >0.5. However, many patients not diagnosed with a co-infection (by blood and respiratory cultures) also had elevated PCT. Overall, the sensitivity and specificity for detection of bacterial superinfection with a PCT cutoff of > 1.5 were only 56% and 84% respectively. There was no difference in clinical characteristics between bacterial co-infection and isolated H1N1 apart from increased radiologic manifestations and increased mortality in the isolated viral infection group (Guervilly et al., 2010).
For now, practice patterns around the use of procalcitonin level to diagnose bacterial infection continue to vary. ATS/IDSA recommend initiation of antibiotics based on clinical judgement regardless of procalcitonin level (strong recommendation), including in influenza-positive patients with clinical and radiographic evidence of CAP (Metlay et al., 2019). There are ongoing placebo-controlled, randomized, double-blind trials to better assess the utility of procalcitonin in lower respiratory tract infection.
Is Procalcitonin Elevated by SARS-CoV-2 Infection?
As outlined above, viral infections typically suppress the cytokines that drive procalcitonin synthesis. In some cohorts of COVID-19, IL-6 levels are higher in respiratory failure non-survivors compared to survivors (Ruan et al., 2020). This could suggest a particular immune response, not classical for viral infection, that could lead to elevated procalcitonin in COVID-19. On the other hand, elevated IL-6 may just be a biomarker of severe ARDS (Meduri et al., 1995; Swaroopa et al., 2016) or bacterial superinfection. Other studies have reported elevated procalcitonin levels in some infected with SARS-CoV-2:
- Guan et al. 2020: PCT > 0.5 ng/mL in 5.5% of patients, 13.7% of severe patients
- Xu et al. 2020: mean 0.04 ng/mL, 11% > 1.0 ng/mL
- Zhou et al. 2020: > 1.0 ng/mL in 30% of patients, significantly higher in non-survivors
Interpretation of these data is difficult as procalcitonin levels were not obtained in all patients, studies used different cutoffs to stratify procalcitonin levels, reference ranges vary between labs, and disease severity varied between studies. Typically, severity was determined from clinical characteristics such as oxygen saturation (Qin et al., 2020; Zhang et al., 2020), need for mechanical ventilation (Guan et al., 2020), or ICU admission (Huang et al., 2020; Wang et al., 2020). In addition, it is unclear how many of these patients had bacterial co-infection. Thus, while patients with severe SARS-CoV-2 infection may have elevated procalcitonin levels, it remains unclear whether this phenomenon reflects viral infection alone, an undiagnosed bacterial infection, or another process.
Bringing It All Together
In summary, there are three possible interpretations of elevated procalcitonin in COVID-19:
- Procalcitonin elevation represents bacterial co-infection that is not always captured by culture data
- Procalcitonin is a marker of severity of ARDS
- Procalcitonin is elevated because COVID-19-associated respiratory failure causes unique immune dysregulation that increases cytokines, such as such as IL-6, which increase procalcitonin synthesis and secretion
As the list above makes clear, there is insufficient specificity associated with elevations in procalcitonin for this to be a useful marker to guide antibiotic therapy in COVID-19. Clinical correlation is advised.
References
- Ahn, S., Kim, W.Y., Kim, S.-H., Hong, S., Lim, C.-M., Koh, Y., Lim, K.S., and Kim, W. (2011). Role of procalcitonin and C-reactive protein in differentiation of mixed bacterial infection from 2009 H1N1 viral pneumonia. Influenza Other Respi. Viruses 5, 398–403.
- Cheng, Z.-B., and Chen, H. (2020). Higher incidence of acute respiratory distress syndrome in cardiac surgical patients with elevated serum procalcitonin concentration: a prospective cohort study. Eur. J. Med. Res. 25, 11.
- Cuquemelle, E., Soulis, F., Villers, D., Roche-Campo, F., Ara Somohano, C., Fartoukh, M., Kouatchet, A., Mourvillier, B., Dellamonica, J., Picard, W., et al. (2011). Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive Care Med. 37, 796–800.
- Guan, W.-J., Ni, Z.-Y., Hu, Y., Liang, W.-H., Ou, C.-Q., He, J.-X., Liu, L., Shan, H., Lei, C.-L., Hui, D.S.C., et al. (2020). Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med.
- Guervilly, C., Coisel, Y., Botelho-Nevers, E., Dizier, S., Castanier, M., Lepaul-Ercole, R., Brissy, O., Roch, A., Forel, J.-M., and Papazian, L. (2010). Significance of high levels of procalcitonin in patients with influenza A (H1N1) pneumonia. J. Infect. 61, 355–358.
- Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., Zhang, L., Fan, G., Xu, J., Gu, X., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506.
- Hui, L., Zhang, X., An, X., Li, J., Zang, K., Shang, F., Zhang, C., and Zhang, G. (2017). Higher serum procalcitonin and IL-6 levels predict worse diagnosis for acute respiratory distress syndrome patients with multiple organ dysfunction. Int. J. Clin. Exp. Pathol. 10, 7401–7407.
- Linscheid, P., Seboek, D., Nylen, E.S., Langer, I., Schlatter, M., Becker, K.L., Keller, U., and Müller, B. (2003). In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology 144, 5578–5584.
- Maruna, P., Nedelníková, K., and Gürlich, R. (2000). Physiology and genetics of procalcitonin. Physiol. Res. 49 Suppl 1, S57–S61.
- Meduri, G.U., Headley, S., Kohler, G., Stentz, F., Tolley, E., Umberger, R., and Leeper, K. (1995). Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 107, 1062–1073.
- Meisner, M. (2014). Update on procalcitonin measurements. Ann. Lab. Med. 34, 263–273.
- Metlay, J.P., Waterer, G.W., Long, A.C., Anzueto, A., Brozek, J., Crothers, K., Cooley, L.A., Dean, N.C., Fine, M.J., Flanders, S.A., et al. (2019). Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 200, e45–e67.
- Müller, B., White, J.C., Nylén, E.S., Snider, R.H., Becker, K.L., and Habener, J.F. (2001). Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J. Clin. Endocrinol. Metab. 86, 396–404.
- Pfister, R., Kochanek, M., Leygeber, T., Brun-Buisson, C., Cuquemelle, E., Machado, M.B., Piacentini, E., Hammond, N.E., Ingram, P.R., and Michels, G. (2014). Procalcitonin for diagnosis of bacterial pneumonia in critically ill patients during 2009 H1N1 influenza pandemic: a prospective cohort study, systematic review and individual patient data meta-analysis. Crit. Care 18, R44.
- Qin, C., Zhou, L., Hu, Z., Zhang, S., Yang, S., Tao, Y., Xie, C., Ma, K., Shang, K., Wang, W., et al. (2020). Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis.
- Rodríguez, A.H., Avilés-Jurado, F.X., Díaz, E., Schuetz, P., Trefler, S.I., Solé-Violán, J., Cordero, L., Vidaur, L., Estella, Á., Pozo Laderas, J.C., et al. (2016). Procalcitonin (PCT) levels for ruling-out bacterial coinfection in ICU patients with influenza: A CHAID decision-tree analysis. J. Infect. 72, 143–151.
- Ruan, Q., Yang, K., Wang, W., Jiang, L., and Song, J. (2020). Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med.
- Schuetz, P., Wirz, Y., Sager, R., Christ-Crain, M., Stolz, D., Tamm, M., Bouadma, L., Luyt, C.E., Wolff, M., Chastre, J., et al. (2017). Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst. Rev. 10, CD007498.
- Swaroopa, D., Bhaskar, K., Mahathi, T., Katkam, S., Raju, Y.S., Chandra, N., and Kutala, V.K. (2016). Association of serum interleukin-6, interleukin-8, and Acute Physiology and Chronic Health Evaluation II score with clinical outcome in patients with acute respiratory distress syndrome. Indian J. Crit. Care Med. 20, 518–525.
- Vidaur, L., Totorika, I., Montes, M., Vicente, D., Rello, J., and Cilla, G. (2019). Human metapneumovirus as cause of severe community-acquired pneumonia in adults: insights from a ten-year molecular and epidemiological analysis. Ann. Intensive Care 9, 86.
- Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., Wang, B., Xiang, H., Cheng, Z., Xiong, Y., et al. (2020). Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA.
- Zhang, J.-J., Dong, X., Cao, Y.-Y., Yuan, Y.-D., Yang, Y.-B., Yan, Y.-Q., Akdis, C.A., and Gao, Y.-D. (2020). Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy.
View all COVID-19 updates
Learn about research in the Division of Pulmonary and Critical Care Medicine