Liquid Biopsy Outperforms Standard Biopsy for Characterizing Acquired Resistance in Gastrointestinal Cancers
Key findings
- In this study, a prospective cohort of 42 patients with molecularly defined gastrointestinal cancer underwent liquid biopsy after they acquired resistance to targeted therapy
- Tumor heterogeneity was substantial: 76% of patients exhibited at least one resistance alteration, 40% of all patients had more than one resistance alteration (range, 2–9) and 78 clinically relevant resistance alterations were detected
- In a subgroup of 23 patients who also had post-progression tissue biopsy results, at least one resistance alteration was identified in 48% by tissue biopsy vs. 87% by liquid biopsy, and multiple resistance alterations were identified in 9% vs. 40%
- Every resistance alteration identified in tissue biopsies was also detected by liquid biopsies, and 78% of patients had resistance alterations identified only by liquid biopsies
- These findings have profound implications for choosing subsequent lines of therapy
When cancer patients become resistant to targeted therapy, the standard approach to selecting the next agent is to order genomic analysis of a core biopsy from one metastatic lesion. However, this approach might underestimate tumor heterogeneity—cells in a tumor can be genetically distinct from one another. It can also fail to account for the fact that multiple resistant subclones sometimes develop concurrently.
Subscribe to the latest updates from Oncology Advances in Motion
Now, Aparna Parikh, MD, MPH, and Ryan Corcoran, MD, PhD, of the Massachusetts General Hospital Cancer Center, and colleagues are routinely using liquid biopsy: analyzing blood samples that contain circulating tumor DNA. In a research letter in Nature Medicine, they report that in a prospective cohort, liquid biopsy was superior to standard biopsy for revealing genetic alterations linked to drug resistance.
Tumor Heterogeneity
Study subjects were 42 patients with gastrointestinal cancers (three tumor types, seven molecular subtypes) who received targeted therapy and achieved partial response or stable disease between September 2014 and March 2018. They underwent liquid biopsy when their cancer progressed:
- 76% of patients exhibited at least one previously validated resistance alteration
- 40% of all patients had more than one resistance alteration (range, 2–9)
- 78 different clinically relevant resistance alterations were detected
Liquid biopsy was able to detect multiple resistance alterations residing concurrently in distinct tumor subclones and different metastatic lesions.
Liquid vs. Standard Biopsy
23 patients also had a tissue biopsy obtained after cancer progression. In this subgroup:
- At least one resistance alteration was identified in 48% of patients by tissue biopsy vs. 87% by liquid biopsy
- Multiple resistance alterations were identified in 9% vs. 40%
- 78% of patients had resistance alterations identified by liquid biopsy that were not detected by tissue biopsy
- No patient had a resistance alteration identified by tissue biopsy alone
Implications for the Clinic
Acquired resistance seems to be characterized by considerable tumor heterogeneity, and the emergence of multiple resistance alterations in an individual patient may be typical, not an exception. These findings have profound implications for choosing subsequent lines of therapy.
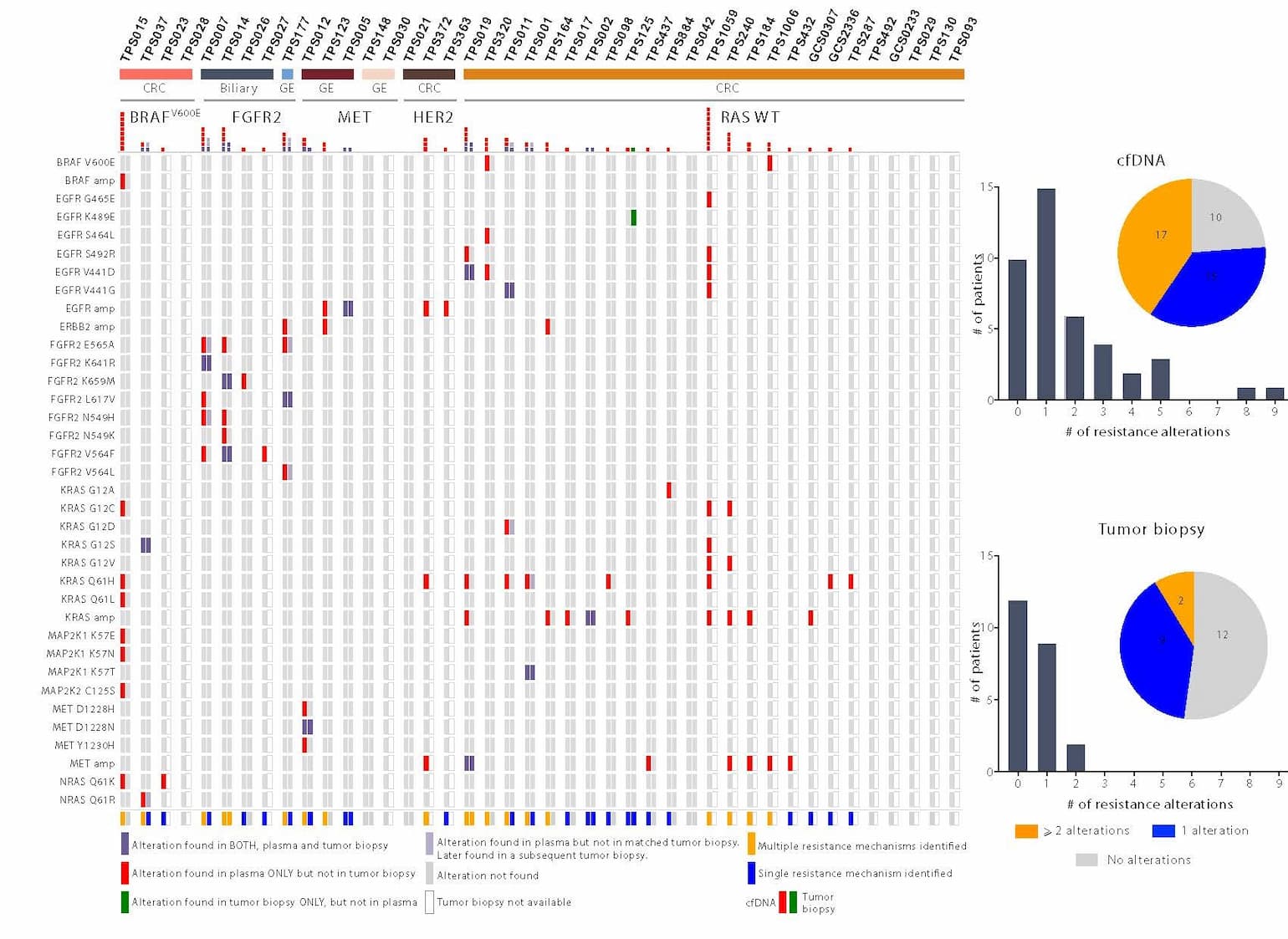
Figure 1: Identification of acquired resistance mechanisms in liquid versus tumor biopsy
A comparison of specific resistance alterations identified in plasma cfDNA (N=42) versus tumor biopsy (N=23) for each patient. Patients are grouped according to tumor type (N=3) (CRC = colorectal, GE = gastroesophageal, biliary) and molecular subtype (N=5) (FGFR2 = FGFR2 fusion, MET = MET amplification, HER2 = HER2 amplification, BRAFV600E, RAS wild type. Red represents alterations identified in plasma, but not in the tissue biopsies; green represents alterations identified in tissue biopsies, but not in plasma; and purple represents alterations identified in both plasma and tissue biopsies. Pale purple represents alterations identified in plasma that were not detected in the post-progression tissue biopsy but were eventually detected in subsequent tissue biopsies from the same patient. The alterations detected in cfDNA versus tumor biopsy are quantified in a histogram across the top of the panel and are summarized graphically on the right, depicting specifically the percentage of patients with one, more than one, or no experimentally validated resistance alterations identified by cfDNA (top) or tumor biopsy (bottom).
view original journal article Subscription may be required
Learn more about the Mass General Cancer Center
Refer a patient to the Mass General Cancer Center