Combination Immunotherapy May Be the Best Treatment for Certain Uterine Cancers
In This Article
- Immunotherapy shows great promise for treating recurring uterine cancers that can otherwise be difficult to treat
- Mass General researchers evaluated patients with six uterine cancer subtypes to identify those with the most potential for immunotherapy treatment
- Endomedtroid-type uterine cancer that is microsatellite unstable and high grade look to be the best candidates for this therapy
Uterine cancer that has recurred after initial treatment poses a major challenge for patients and physicians because it is largely unresponsive to many of the therapies traditionally used in the clinical setting. Fortunately, immunotherapy is showing great promise in uterine cancer, just as it is in other types of cancer.
Subscribe to the latest updates from OB/GYN Advances in Motion
However, much work is still needed to understand which patients with uterine cancer will benefit from immunotherapy and what drug combinations might be most effective. At Massachusetts General Hospital, this research is being spearheaded by Amanda Ramos, MD, a gynecologic oncology fellow in the Department of Obstetrics and Gynecology, Whitfield Growdon, MD, a specialist with the Center for Gynecologic Oncology in the Mass General Cancer Center and the Department of Obstetrics and Gynecology.
Checkpoint Inhibitor Therapy
To appreciate the advances that Dr. Ramos, Dr. Growdon and colleagues are making, it’s necessary to understand immune checkpoint inhibitor therapy.
In a healthy person, T cells upregulate expression of cell-surface proteins called immune checkpoints, which help to control immune response. When engaged with their ligands, checkpoint proteins serve as a physiologic “brake” on the immune system. This prevents unchecked inflammation and autoimmunity.
In a cancer patient, however, braking can allow tumor cells, which would normally be recognized by T cells, to evade the immune system. Checkpoint inhibitor drugs block the checkpoint protein’s braking action, making it more likely that the immune system will recognize the tumor cell and attack it. An important example of these drugs is pembrolizumab, a monoclonal antibody that targets PD-1, a checkpoint protein on the membrane of T cells.
“Immune checkpoint therapy has proven effective in many malignancies, including lung cancer and melanoma,” says Dr. Ramos. “Understanding the immune landscape of gynecologic cancers will be important in moving forward with research in this organ system.”
The Influence of Microsatellite Instability
Cancers of the uterus are genetically complex, so not all patients with uterine cancer respond to pembrolizumab in the same way. About one-third of these cancers harbor high levels of mutations in DNA repair genes, a phenomenon known as “microsatellite instability” or “genomic instability.”
This is advantageous for checkpoint inhibitor therapy, because a tumor that is microsatellite unstable expresses a large amount of abnormal proteins for the immune system to recognize and attack. For example, a previous clinical trial showed that patients with microsatellite-unstable uterine tumors had a 53% objective response rate to pembrolizumab. In contrast, in patients with uterine cancers lacking microsatellite instability, the response rate was only 13%.
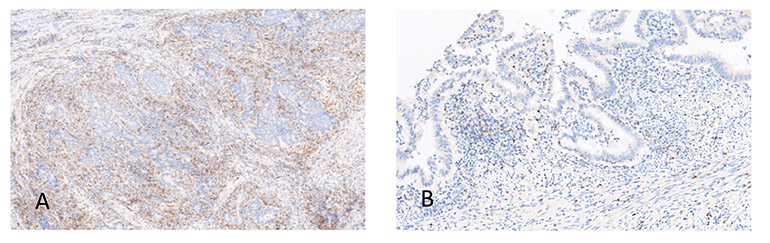
Fig. 1: PD-1 Expression
Image A shows a high PD-1 expressor. Image B shows a low PD-1 expressor.
One of Many Checkpoint Proteins
Besides PD-1, multiple other checkpoint proteins exist on the surfaces of T cells and tumor cells (see Figure 1). Other examples include TIM-3, LAG-3 and CTLA-4. The individual and simultaneous expression of these checkpoint proteins has yet to be explored in the setting of uterine cancer.
It seems possible, however, that they represent additional therapeutic targets that could potentiate the anti-tumor response seen in patients with microsatellite instability and perhaps in all patients with cancer of the uterus.
The Research Initiative
Dr. Ramos, Dr. Growdon and colleagues are investigating the immune environment of uterine cancer cells and associated immune cells to identify possible targets for combination immune therapies. They are focusing on PD-1, TIM-3, LAG-3 and CTLA-4, since checkpoint inhibitor drugs that target those proteins are already available or in development.
“Our hope is that through this research, we can identify immune checkpoints that are upregulated simultaneously in the endometrial cancer immune environment as potential targets for combined immune therapies,” says Dr. Growdon.
In the first phase of this project, the researchers analyzed a group of patients with tumors from six uterine cancer subtypes. They used a biochemical assay based on branched DNA signaling technology to detect and quantify RNA expression in the checkpoint proteins of interest. The cancer tissue was then stained with antibodies to these proteins to confirm the presence of the immune checkpoints and immune cells (see Figure 2).
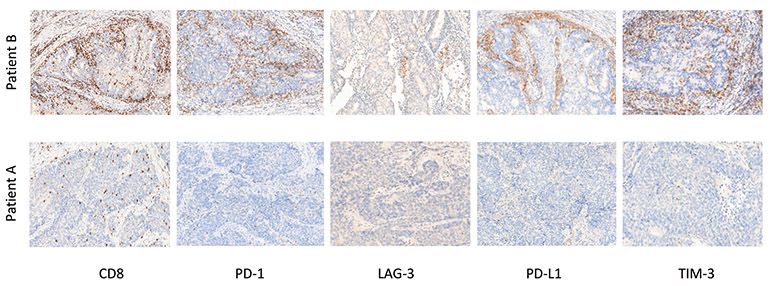
Fig. 2: High-grade Endometrioid Patient Cohort Co-expressors
Images shown are from two different patients included in the study cohort.
Study Results
These procedures have already yielded several interesting findings:
- RNA expression specific to T cells known to harbor PD-1 was highest in patients with endometrioid types of uterine cancers, the most prevalent of the uterine carcinomas (see Figure 3). This implies that endometrioid cancers are readily detected by the immune system and may have multiple mechanisms of immune system evasion
- A high level of T-cell infiltration was observed around the boundary of endometrioid tumors, with some cells appearing to penetrate the cancer itself. In this situation, T cells became exhausted through increased expression of multiple inhibitory checkpoint proteins, specifically PD-1, TIM-3 and CTLA-4. Consequently, the T cells were rendered less effective in combating the cancer
- T-cell exhaustion was fueled by the ability of the tumor cell to express ligands, such as PD-L1, that can activate the T-cell inhibitory checkpoints
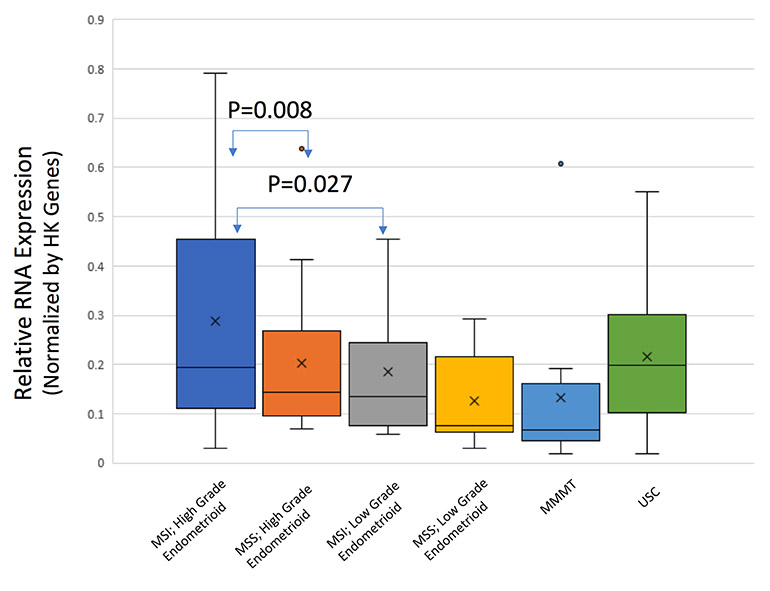
Fig. 3: RNA Expression by Tumor Type
PD-1 RNA expression is elevated in MSI, high-grade endometrioid cancers.
Potential Candidates for Combination Immunotherapy
An important additional finding was that T-cell exhaustion signatures are enriched in endometrioid cancer that is microsatellite unstable and high grade. Patients with that type of uterine cancer thus seem likely to benefit from combinations of checkpoint inhibitor therapy. In contrast, carcinosarcomas and uterine serous carcinomas displayed a lack of microsatellite instability and immune checkpoint expression, which may help to explain their aggressive nature.
The researchers call for clinical trials of combination checkpoint inhibitor therapy for patients with endometrioid-type uterine cancer that is microsatellite unstable and high grade.
“Throughout the nation, there are several trials assessing tumor response to the combination of immune checkpoint inhibitors with other therapies such as chemotherapies,” Dr. Ramos says. “Looking forward, our team will be investigating the immune landscape of endometrial cancer recurrences and cervical cancers with the hope of identifying more therapeutic targets in these cancers that are difficult to treat.”
Learn more about the Center for Gynecologic Oncology
Learn more about the Department of Obstetrics & Gynecology