Pathways Case Record: Idiopathic Achalasia in the Setting of Autoimmune Disease
In This Case Study
- A 41-year-old man presented to the clinic with a year and a half history of progressive difficulty swallowing solids then liquids, a 6- to 8-month history of daily vomiting, and 80-pound weight loss
- A month later, he experienced worsening weakness, shortness of breath, and continued difficulty swallowing with non-bloody, nonbilious vomiting
- Labs on admission were significant for elevated creatinine and blood urea nitrogen levels, and computed tomography showed left kidney atrophy without hydronephrosis, esophageal dilation, and an enlarged heart with small pericardial effusion
- His symptoms and further evaluation were consistent with the swallowing disorder called type I achalasia
- The Pathways Consult Service was consulted to investigate the etiology of this patient's achalasia, including which component of the immune system is driving the disorder and what is the infectious trigger of idiopathic achalasia
A 41-year-old man with a history of psoriasis, ulcerative proctitis, and lack of recent contact with the healthcare system presented to the clinic with a year and a half history of progressive difficulty swallowing solids then liquids, a 6- to 8-month history of daily vomiting, and an accompanied 80-pound weight loss. He had no significant travel history or known family history of autoimmune disease, kidney disease, or malignancy. Vital signs were notable for high blood pressure (220/130). His initial labs were significant for microcytic anemia, anion gap metabolic acidosis, and renal failure. He was instructed to present to the emergency department but declined.
Subscribe to the latest updates from Advances in Motion
A month later, he experienced worsening weakness, shortness of breath, and continued difficulty swallowing (termed dysphagia) with non-bloody, nonbilious vomiting. Labs on admission were significant for elevated creatinine and blood urea nitrogen (BUN) levels. Computed tomography showed left kidney atrophy without hydronephrosis, esophageal dilation, and an enlarged heart with small pericardial effusion. His symptoms and further evaluation were consistent with the swallowing disorder called type I achalasia.
The Pathways Consult Service in the Department of Medicine at Massachusetts General Hospital was consulted to investigate the etiology of this patient's achalasia. The Pathways team's investigation focused on three key questions:
- Where is the primary neuromuscular defect in achalasia?
- Which component of the immune system is driving achalasia?
- What is the infectious trigger of idiopathic achalasia?
Background and Diagnosis
Achalasia is characterized by loss of or abnormal involuntary constriction and relaxation of muscles in the distal esophagus and failure of the lower esophageal sphincter (LES) to relax, which manifests as progressive dysphagia to solids and liquids, regurgitation of undigested food or saliva, and eventually weight loss. While most cases are classified as idiopathic, an estimated 1.4%–5.4% of patients with achalasia have an identifiable cause (secondary achalasia), such as malignancy (most common), postoperative complication, and amyloidosis (Annals of Esophagus). Given our patient's medical history, he likely presents with 'idiopathic' achalasia.
Idiopathic achalasia is associated with the loss of inhibitory neurons in the myenteric plexus of the esophagus (Eur J Clin Invest). These inhibitory motor neurons act intrinsically via a reflex arc and are activated by mechanical stresses (Am J Physiol Cell Physiol, Am J Physiol Gastrointest Liver Physiol, Am J Physiol Gastrointest Liver Physiol). However, they are regulated upstream by vagal preganglionic motor neurons from the dorsal motor nucleus. Loss of connected neurons can lead to neuronal cell death as well as changes in neuron-intrinsic factors (e.g., neurotrophic molecules or inhibitory factors). It is unknown whether achalasia is due to a pure loss of these inhibitory neurons or if upstream signaling is also affected. The severe hypertension observed in our patient may suggest upstream autonomic dysregulation. Surgical vagotomies and extra-esophageal autonomic dysfunction have been linked to achalasia (Dig Dis Sci, PLoS One). The depth of biopsies required to reach the myenteric plexus presents a major challenge to studying the impact of inhibitory neuron loss in the enteric nervous system. While non-invasive imaging modalities (e.g., fMRI) may inform upstream neuronal signaling changes, this challenge highlights the need to develop new diagnostic and research tools to assess neuronal activity within the gastrointestinal tract.
Although the loss of inhibitory myenteric neurons is common in achalasia, the etiology of neuron loss remains elusive. Notably, mucosal and submucosal immune invasion of the distal esophagus and LES is seen in all clinical classifications of achalasia. Immunohistochemical analysis of affected LES tissue demonstrates an enrichment of subsets of CD4+ T cells including Th22 and Th17 cells. Furthermore, Th22 and Th17 lineage-specific cytokines such as IL-17A, IL-17F, and IL-21 are increased in LES tissue of achalasia patients compared to controls (J Immunol Res). These cytokines and helped T cell subsets are also enriched in autoimmune diseases such as Sjogren's, multiple sclerosis, rheumatoid arthritis, juvenile diabetes, autoimmune uveitis, and importantly in reference to our patient, psoriasis, and ulcerative colitis (Annu Rev Immunol, Inflamm Bowel Dis). While the question remains if Th22 and Th17 immune cell invasion serves as a cause of neuron dysfunction or is merely a response to an already disrupted autonomic neuropathy, the correlation of these T cell subsets and primary autoimmune dysfunction is clearly established.
Given this background, next, we asked: what may be a trigger to such an autoimmune response? Recent advances in other autoimmune disorders such as multiple sclerosis have suggested a critical role of viral triggers. This may represent a parallel for the pathophysiology of autoimmune achalasia. In a study of LES tissue samples, HSV-1 was detected, and active viral replication was demonstrated in all samples from achalasia patients but not in controls (J Immunol Res). In another study, two HSV-1 encoded miRNAs, hsv1-miR-H1 and hsv1-miR-H18, were found to be differentially expressed in LES muscle biopsy samples from patients with achalasia compared to controls (Dig Endosc). Examination of the immune response to HSV-1 in patients with achalasia revealed that local lymphocyte populations in the LES of patients responded to stimulation with HSV-1. They showed an increase in proliferation, as well as IFNγ and IL-2 secretion at 5 days (Am J Gastroenterol). Moreover, no such changes are elicited by stimulation with other viruses (i.e., CMV, EBV, AdV).
The inflammatory infiltrate in achalasia is predominated by Th17 CD4+ T cell populations. Interestingly, a significant Th17 response has been noted in other syndromes related to HSV-1 infection, including herpes keratitis (J Immunol). In rabbits that develop keratitis in response to HSV-1 infection, a Th1-type cytokine milieu predominates early in the cornea, but a Th17-type milieu emerges throughout disease development. In fact, the ratio of IFNγ+ Th1 cells to IL-17+ cells decreased from 10:1 to 3.4:1 between day 8 and day 21. Moreover, anti-IL-17 therapy and IL-17 deletion decrease lesion severity. A similar role for the Th17 effector arm has been suggested in patients with recurrent herpes labialis (RHL). One study demonstrated a significant increase in peripheral blood Th17 differentiated cells in patients with RHL compared to controls (Int J Immunopathol Pharmacol).
Achalasia and other disorders of the myenteric plexus rarely co-occur, suggesting some unique characteristics of the neuronal innervation of the LES. However, whether there is a lack of human herpesvirus tropism for more distal neurons, differences in proteome resulting in a lack of specific autoepitopes or susceptibility to cytopathic effect, or simply regional infection of neurons because of specific routes of inoculation remain unknown. After orogastric inoculation of mice with HSV-1, viral DNA was detected in the myenteric plexus of the ileum and colon for up to 10 weeks (Front Cell Infect Microbiol). Another study showed that the spread of herpesvirus from genital inoculation can result in megacolon in mice, with similar histopathologic findings to achalasia in the myenteric plexus (Cell Host Microbe). Importantly, HSV-1 in these mice was detected in the urinary bladder, paracervical ganglia, and large intestine but was not detected in the terminal ileum, liver, or sympathetic chain. Taken together, these results demonstrate there is both viral tropism and pathologic effect distally, suggesting the route of inoculation is important for the development of neurogastrointestinal disease secondary to HSV-1 infection.
Summary and Future Steps
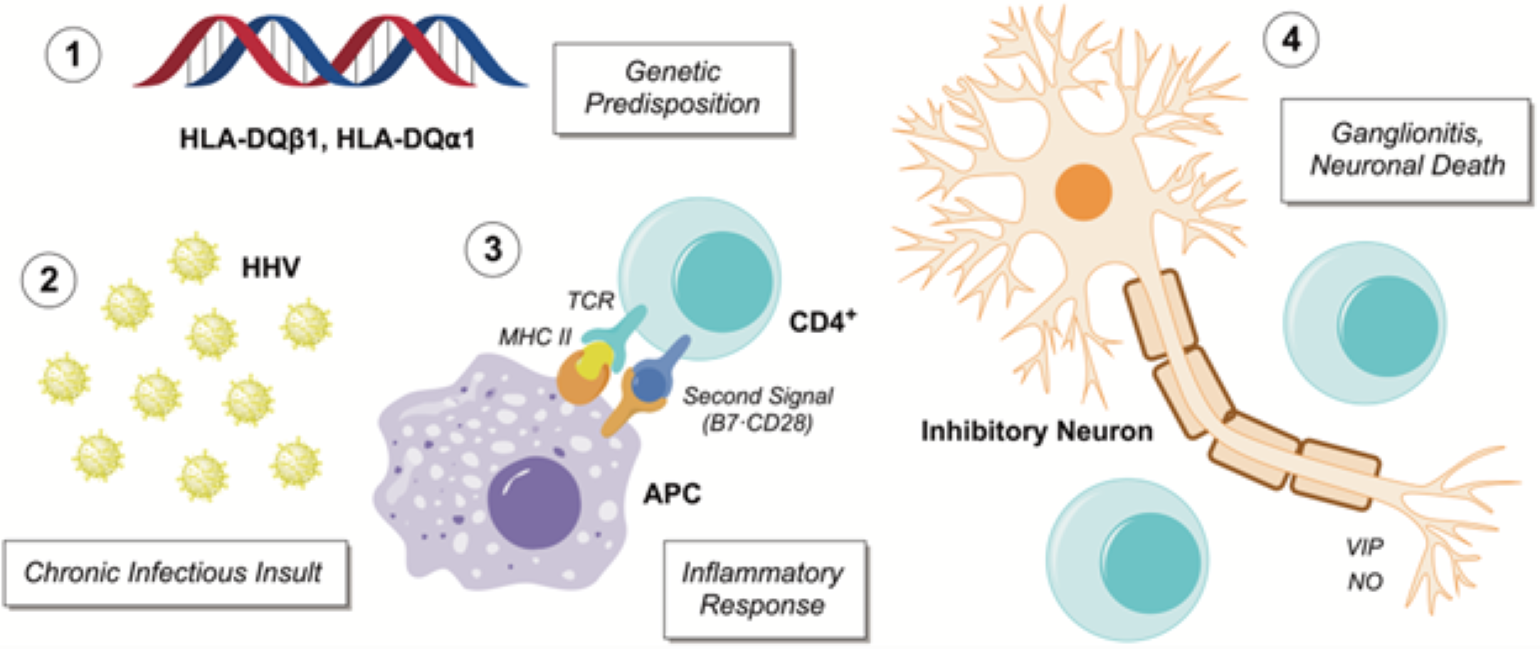
Figure 1
Proposed autoinflammatory model of achalasia from the Pathways Consult Service.
While we are far from fully understanding this patient's overall presentation, the Pathways Team hypothesizes that the patient's autoimmune disorders, exacerbated by a viral infection, likely play a role. We hypothesize that the loss of inhibitory neurons in achalasia is immune-mediated and triggered by HSV-1 or another herpesvirus infection (Figure 1). We propose five experiments to investigate this hypothesis.
- To investigate potential autoantigens, we propose using slides of myenteric neurons to identify potential autoantibodies unique in the patient sera as well as characterization of the patient's peripheral blood mononuclear cells and possible reactivity to esophagus-specific proteins. If an autoantigen is identified, a murine model could be utilized to determine if achalasia or at least neuron-specific cytotoxicity ensues.
- We propose a BLAST search of candidate peptides against the proteomes of human herpesviruses to allow for targeted homologous viral peptide library generation, and patient T cell cross-activation.
- We propose a pulldown experiment using patient serum against viral lysates, and the identified self and viral proteins could be probed for 3-dimensional similarities using crystal structures, if available, or a structural prediction software such as AlphaFold.
- Leveraging the work done in the multiple sclerosis field (Science), identification of an extrinsic trigger by HSV-1 could be pursued by examining seroconversion against a series of human herpesviruses in achalasia patients versus matched healthy controls.
- Examine the development of autoimmune neurogastrointestinal disease after oral inoculation with human herpesvirus versus adenovirus (or other inactive virus) control in an attenuated model, MRL/++, that has been used to probe hypothesized triggers of autoimmunity (J Immunol).
In summary, we propose further immunologic studies to investigate an autoimmune etiology of achalasia and to identify potential targets and triggers. This work could allow us to identify targeted therapeutics for patients with achalasia, whether via the immune system or viral replication.
Learn about the Pathways Consult Service
Refer a patient to the Pathways Consult Service