Pathways Case Record: Small Fiber Neuropathy and Recurrent GI Infections
In This Case Study
- A 24-year-old woman presented with recurrent blood infections with bacteria primarily found in the gut
- She developed a complex series of symptoms starting at age 15 when she began to have recurrent nausea, vomiting, and fainting episodes
- The woman was diagnosed with postural orthostatic tachycardia syndrome (POTS), small fiber neuropathy (SFN), and impaired gastrointestinal (GI) motility
- She tried several medications and received an experimental stem cell transplant, after which she developed severe infections requiring multiple prolonged hospitalizations without significant improvement
- During one of these hospitalizations, the Pathways Consult Service was consulted and focused on the relationship between the patient's neuropathy and recurrent GI bacterial infections
A 24-year-old woman presented with recurrent blood infections with bacteria primarily found in the gut. She developed a complex series of symptoms starting at age 15 when she began to have recurrent nausea, vomiting, and fainting episodes. She was diagnosed with postural orthostatic tachycardia syndrome (POTS), small fiber neuropathy (SFN), and impaired gastrointestinal (GI) motility.
Subscribe to the latest updates from Advances in Motion
The woman tried several medications and received an experimental stem cell transplant. Following the transplantation, she developed severe infections that required multiple prolonged hospitalizations without significant improvement. During one of these hospitalizations, the Pathways Consult Service in the Department of Medicine at Massachusetts General Hospital was consulted and focused on the relationship between the patient's neuropathy and recurrent GI bacterial infections. We considered four questions:
- Does this patient have small fiber neuropathy affecting her GI tract?
- Does this patient have disrupted gut integrity?
- Does small fiber neuropathy lead to GI immune dysfunction?
- Could immune dysfunction lead to the disruption of intestinal barrier integrity and susceptibility to GI infection?
Background and Diagnosis
The human nervous system is made up of multiple nerve types and has three main branches: the sympathetic (fight or flight), parasympathetic (rest and digest), and the enteric nervous system. Small nerve fibers (e.g., type Aδ fibers and C fibers) play a role in detecting heat, low pH (acidity), and pain. Small fiber neuropathy occurs when these fibers are damaged. The gold standard for diagnosis is a biopsy (typically a skin biopsy) and quantification of A/C fibers based on age- and sex-matched controls. Within the GI system, neuropathy is difficult to diagnose because the nerve plexus of interest lies deep within the muscular layer of the gut, where full-thickness biopsies carry a risk of life-threatening complications such as strictures, perforation, and infection.
Dysregulation of small fiber nerves may result in impaired blood flow regulation in the gut, diminishing the gut's ability to react and perfuse and contributing to GI dysmotility (JAMA Neurol). This dysmotility may affect gut permeability, but research in this area has been difficult due to variability in environmental conditions and the feasibility of making accurate measurements in living organisms. Recently, there has been a push to standardize the protocol for measuring gut permeability using high-performance liquid chromatography to measure consumed 13C-mannitol and lactulose to distinguish between transcellular (through gut cells) and paracellular (around gut cells) permeability (Gastroenterology).
Understanding the relationship between the immune system in the gut and the small fiber neurons that innervate it will be critical to understanding the pathophysiology in our patient. Research has begun to unravel the complicated relationship between host defense systems and gut innervation, with emerging evidence describing crosstalk between the immune system and neurons (Mucosal Immunol). A recent paper suggests that disruption of the gut nervous system leads to enteric immune dysregulation using a Salmonella infection model (Cell). The authors observed impaired release of CGRP, a common neuropeptide, in mice with denervated enteric neurons, which increased susceptibility to Salmonella infection. Additional studies have demonstrated that neuropeptides secreted by nerves in the GI tract protect and/or increase susceptibility to gut infections by regulating innate lymphoid cells (Nature, Front Immunol). Innate lymphoid cells 3 (ILC3s) are critical players in maintaining mucosal immunity via the secretion of IL-22 and IL-17, which trigger the secretion of antimicrobial peptides and mucin production (Front Immunol).
Given that common neuropeptides may regulate ILC3s, it is possible that impaired nerve function (such as in small fiber neuropathy) could lead to a diminished release of neuropeptides and a subsequent impact on ILC3 function. A deeper understanding of immune dysregulation due to nerve impairment may help identify novel therapeutic targets for our patient. For example, zonulin, a modulator that reversibly regulated intestinal permeability, was discovered by studying cholera proteins. Further insights into the mechanism resulted in developing a zonulin antagonist that has shown promise in maintaining gut integrity in different pathologies (Lancet).
Based on current literature and our patient's history, our central hypothesis is that the small fiber neuropathy contributed to gut immune dysfunction and impaired barrier integrity, leading to increased susceptibility to GI-mediated bacterial infections. Evidence continues to build, suggesting that small fiber neuropathy can affect GI motility, GI barrier integrity, and immune function. We face challenges in directly quantifying neuropeptide levels within the mucosa and correlating these levels to ILC3 function due to the depth required to perform biopsies for these measurements. A more feasible approach is to target downstream effects of neuronal dysfunction by focusing on ILC3s since they are located more superficially within the GI wall. We hypothesize that as a consequence of small fiber nerve dysfunction, neuropeptide production and/or release is diminished, leading to impaired ILC3 production and function. Additionally, this nerve dysfunction could disrupt intestinal wall integrity by downregulating tight junction proteins.
Summary and Future Steps
Further investigations into the contributions of small fiber neuropathy to altered GI integrity and function are warranted. Key experiments to better understand the pathophysiology present in our patient include:
- Analysis of gut ILC3s using pre-existing intestinal mucosal biopsies using flow cytometry and immunofluorescent microscopy to characterize ILC3 with markers such as RORγ, IL-17Rα, and CD3ε as well as RT-PCR to quantify IL-17 and IL-22 compared to healthy controls
- Mouse modeling of small fiber neuropathy (similar to Cell) to measure ILC3 quantity and function, GI tract biopsies, and gut barrier integrity
- Establishing a repository of enteric full-thickness biopsies for future comparisons of reduced density of neurons in patients
- Assessment of gut permeability in our patient through consumption of 13C-mannitol and lactulose (Figure 1). Understanding the intimate and complex relationship between small fiber neuropathy and GI function could enable the identification of new therapeutic targets to control GI immunity and maintain gut integrity to prevent recurrent life-threatening infections
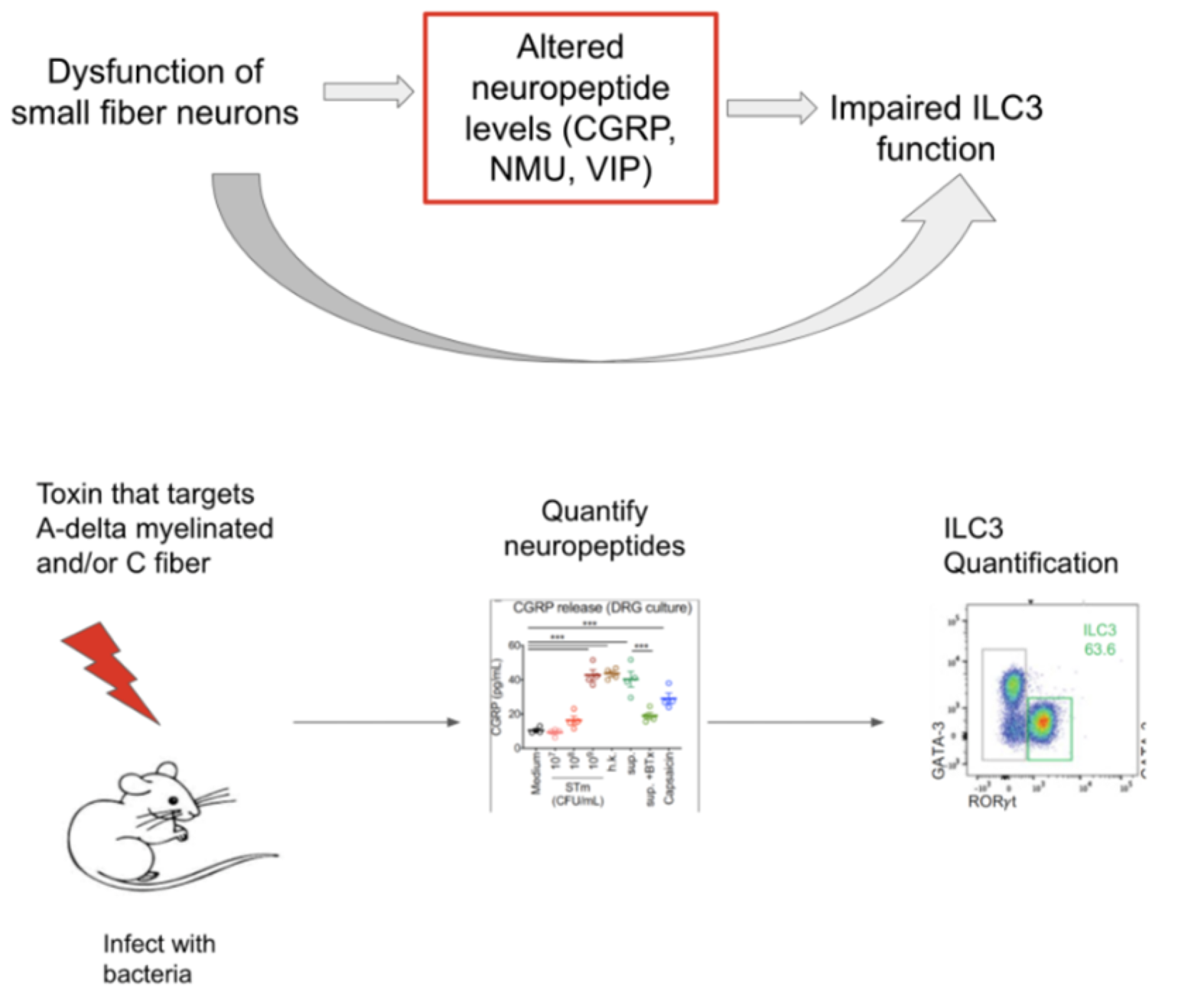
Figure 1
Learn more about the Pathways Consult Service at Mass General
Explore research in the Department of Medicine