Pathways Case Record: Anti-HMGCR+ Immune-mediated Necrotizing Myopathy
In This Case Study
- An 85-year-old woman was admitted for progressive muscle weakness and fatigue over the course of a month
- A muscle biopsy showed pathology consistent with immune-mediated necrotizing myopathy
- Furthermore, she had a high titer of antibodies directed against HMG CoA reductase (HMGCR), elevated cardiac troponin, and evidence of myocarditis with late-gadolinium enhancement on cardiac MRI
- The Pathways Service was consulted and focused on the potential of cells infiltrating the necrosing muscle and HMGCR auto-antibodies
An 85-year-old woman was admitted for progressive muscle weakness and fatigue over the course of a month. 2.5 years prior to admission, she had an ST-segment elevation myocardial infarction (MI) that required placement of a drug-eluting stent and initiation of dual antiplatelet therapy and high dose atorvastatin (80 mg daily). However, she stopped taking these medications after about three months. Shortly before admission, she developed a pruritic rash across her torso and arms, which resolved following oral prednisone treatment. She required assistance by a walker and fell multiple times due to weakness.
Subscribe to the latest updates from Advances in Motion
Upon admission, she had notable proximal, symmetric muscle weakness, myoglobinuria, and severely elevated creatine kinase, indicating pronounced muscle injury. A muscle biopsy showed pathology consistent with immune-mediated necrotizing myopathy (IMNM). Furthermore, she had a high titer of antibodies directed against HMG CoA reductase (HMGCR), elevated cardiac troponin, and evidence of myocarditis with late-gadolinium enhancement on cardiac MRI. She was initially treated with high-dose steroids and discharged to rehab and then home but was readmitted due to worsening weakness. During this second admission, she was treated with high-dose steroids and intravenous immunoglobulin for five days, which resulted in significant improvement in her labs and clinically improved muscle strength.
The Pathways Consult Service at Massachusetts General Hospital was consulted and focused on the potential of cells infiltrating the necrosing muscle and HMGCR auto-antibodies, which drove two questions:
- Is the myocyte necrosis mediated by anti-HMGCR IgG binding HMGCR on myocytes and locally activating complement and/or antibody-dependent cellular cytotoxicity (ADCC)?
- What role did statin therapy play in the initiation of IMNM?
Background and Diagnosis
IMNM is an autoimmune disease that typically presents with severe proximal weakness, myofiber necrosis with little infiltration by immune cells, and is often associated with muscle-specific antibodies (anti-HMGCR or anti-SRP). On pathology, the disease is characterized by macrophage invasion and engulfment of necrotic muscle, sparse T cells, and complement fixation. Relapses are frequent, and there are few clinical studies to assess optimal treatment paradigms. This disease is rare (2-3 in 100,000 person-years in patients treated with statins) and is treated with broad immunosuppression (N Engl J Med).
It is currently unknown how statins cause IMNM and what immune components mediate myopathy. The association of the myopathy with statin use or exposure to natural statin-like compounds (e.g., red yeast rice) indicates that statins, which lower cholesterol levels by inhibiting the enzymatic activity of HMGCR, are likely involved in inciting disease, potentially because statins upregulate HMGCR to a degree that may contribute to loss of immune tolerance (Arthritis Rheum). While anti-HMGCR antibodies are associated with disease, it is unknown whether they are necessary or sufficient for pathology. A mouse model demonstrated that IgG from a patient with anti-HMGCR IMNM reproduced muscle weakness, which suggests the antibodies are sufficient to cause disease (Ann Rheum Dis). However, a case study found that three patients treated with IVIg monotherapy had persistent anti-HMGCR antibody titers despite the resolution of the disease clinically (N Engl J Med). Furthermore, plasmapheresis is clinically ineffective in anti-HMGCR IMNM, while it is comparatively therapeutic in diseases with known pathogenic antibodies such as myasthenia gravis (personal communication, E. Tiniakou).
Since complement binds to myocytes more commonly in IMNM than in other inflammatory myositis (Neurology), it is also thought that anti-HMGCR antibodies may cause myocyte necrosis through local activation of the classical complement pathway. However, C5 convertase blockade (i.e.,eculizumab) and plasmapheresis are clinically ineffective (personal communication, E. Tiniakou), arguing against complement-mediated necrosis. With regards to T cell-mediated immunity, there is a very strong association of HLA-DRB1*11:01 with anti-HMGCR IMNM, whereby patients with the disease have relative odds of having this haplotype on the order of ~25-50 (Arthritis Care Res [Hoboken]). It is unknown how this HLA haplotype (as part of a major histocompatibility complex [MHC] class II molecule) contributes to pathogenesis, and there have been limited studies to date interrogating the functional role of T cells in the disease pathophysiology.
Our patient presented with subacute, painless, proximal muscle weakness typical of anti-HMGCR IMNM with the exception of her myocarditis, which was a curious finding given that this is not previously reported in association with IMNM. We hypothesize that CD4+ T cells recognize HMGCR via a T cell receptor (TCR)-MHC class II interaction that is more likely in statin exposed, susceptible hosts who have the HLA-DRB1*11:01 haplotype. Loss of immune tolerance occurs because statins sterically block and increase expression of HMGCR, leading to amplified uptake of HMGCR by tissue-resident antigen-presenting cells in muscle and presentation to naïve CD4+ T cells. Subsequently, activated CD4+ T cells that recognize the HMGCR antigen stimulate B cells to increase the production of anti-HMGCR IgG, activating the complement pathway. Although the role of macrophages in IMNM pathophysiology remains less understood, these activated T cells could stimulate macrophage activation leading to necrosis via phagocytosis and antibody-dependent cellular cytotoxicity.
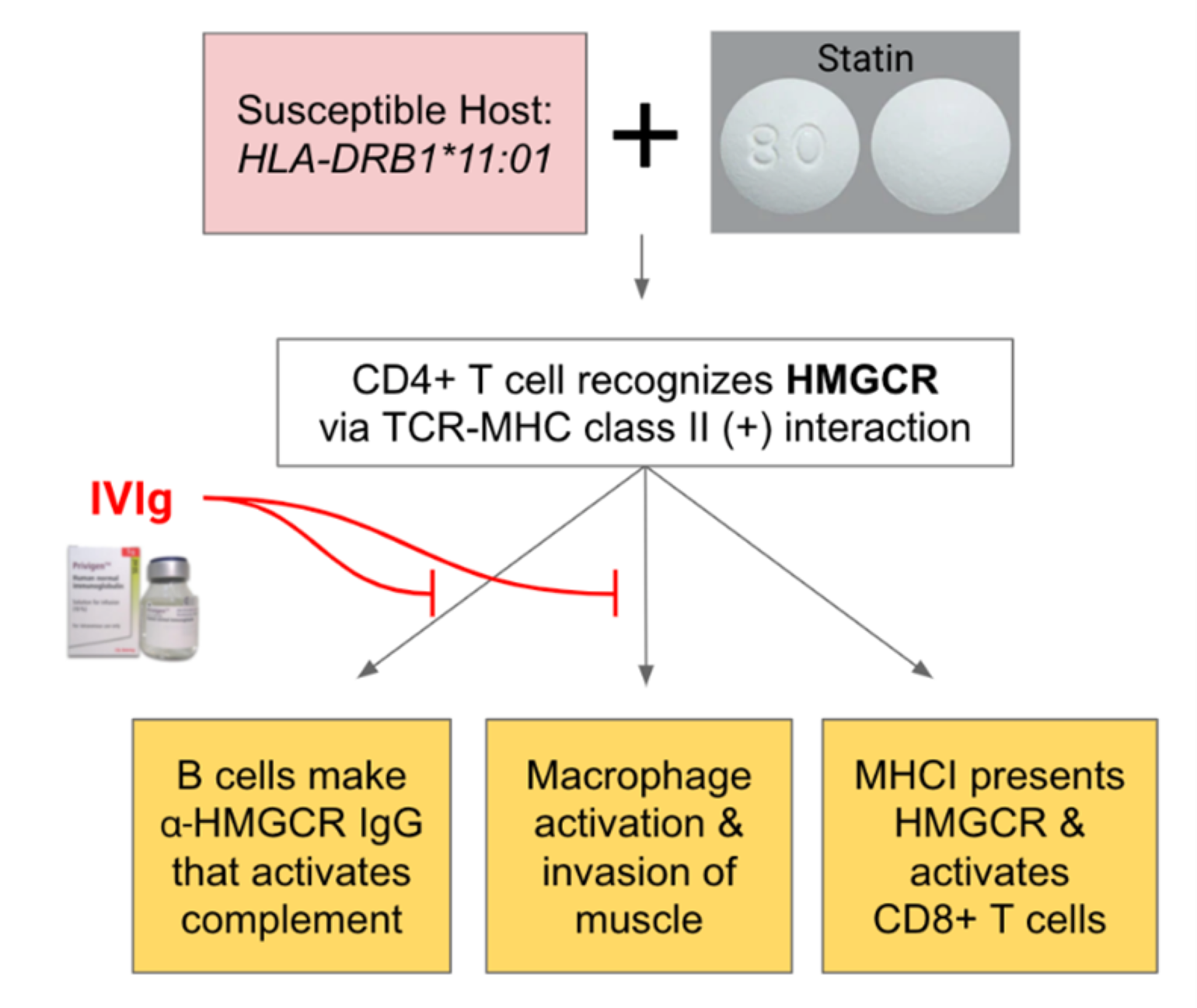
Figure 1
Proposed mechanism of action for our patient's IMNM and potential treatment strategy with IVIg.
Based on current literature and above hypothesis, we predict that IVIg would be the most effective therapy for our patient. We suggest based on two primary reasons: (1) sialic acid residues on anti-HMGCR antibodies from immunoglobulin donors bind to inhibitory receptors that dampen immune responses in patients, and (2) occupation of neonatal Fc receptor (FcRN) with IVIg leads to endocytosis and clearance of endogenous antibodies, including pathologic anti-HMGCR IgG (Proc Natl Acad Sci U S A). Improved treatment strategies for IMNM may also include the generation of an HMGCR 1-methylpseudouridine (m1Ψ) mRNA tolerizing vaccine to increase T regulatory (Treg) cells that have specific TCR sequence for HMGCR, as well as chimeric antigen receptor Treg cells with specificity for muscle cells that could tolerize the tissue from immune attack. A m1Ψ mRNA vaccine for another autoimmune disease, encephalomyelitis, shows promise in experimental models (Science). Together, the Pathways Team hypothesizes that a combination of IVIg and HMGCR m1Ψ mRNA would be effective to treat IMNM by targeting the excess of autoantibodies and T cell mediated immunopathology (Figure 1).
Summary and Future Steps
Further investigations into the underlying mechanism of IMNM are warranted. Key experiments to better understand the pathophysiology include:
- Characterization of T cell repertoire of peripheral blood from IMNM patients through single-cell RNA sequencing with paired TCR analyses
- Combined proteomics and transcriptomics to determine if HMGCR is alternatively spliced or post-translationally modified in human tissue and if statins affect this process
- Develop an experimental model (HLA-DRB1*11:01 knock-in mouse) to examine the impact of statin exposure, muscle injury, immune checkpoint blockade, and the role of specific immune cells (e.g., B cells, macrophages) in disease progression
- Utilize TCR sequences identified in #1 to determine if specific T cell clones can recognize and activate after binding to MHC class II-HMGCR complex, and if so, determine which epitope of HMGCR is presented by utilizing an in vitro system
These studies could delineate the biology of IMNM and lead to new therapeutic insights.
Learn more about the Pathways Consult Service at Mass General
Explore research in the Department of Medicine