Lung Pathology in COVID-19
The FLARE Four
- Clinical lung injury and ARDS can be associated with a variety of histologic patterns
- In COVID-19, the limited available histologic evidence predominantly reveals classic DAD with very little organization and fibrosis (at least in the acute setting)
- AFOP has been described in one small case series; however, definitive diagnosis is hampered by sampling techniques. Additionally, there are a multitude of other pathologic findings noted in multiple other COVID-19 post-mortem series
- There are unanswered questions regarding the timing of lung injury and development of DAD, and there is a dearth of data regarding histological correlates in patients who recover from SARS-CoV-2 infection
Many people are wondering...do the lung pathologists know what kind of disease is caused by SARS-CoV-2?
Subscribe to the latest updates from FLARE Advances in Motion
Introduction
Both in the scientific literature and on social media, it has been alleged that respiratory failure in COVID-19 represents a novel and unique entity. Prior FLAREs have reviewed clinical case series of COVID-19 patients with respiratory failure, which revealed moderate-to-severe hypoxemia and reduced compliance consistent with ARDS. Nevertheless, the possibility remains that, at a tissue level, the lungs of patients with severe COVID-19 could reveal a new or unique pattern of injury. Tonight, we examine COVID-19 lungs under a microscope (pun intended). In this FLARE, we will review the common histologic patterns of acute lung injury and compare these to the available pathologic cases in COVID-19.
What Are the Histologic Patterns Associated With Acute Lung Injury?
Acute lung injury (ALI) has a wide variety of etiologies, including infections (viral, bacterial, fungal), toxic ingestions, drug reactions, systemic disease (i.e. collagen vascular disease), alveolar hemorrhage, and radiation. Furthermore, ALI from any of these etiologies may be associated with a variety of histologic manifestations, which include:
1) diffuse alveolar damage (DAD)
2) acute fibrinous and organizing pneumonia (AFOP)
3) organizing pneumonia (OP)
These patterns can overlap with one another and may in fact represent a continuum.

Slide courtesy of Dr. Hariri.
1. Diffuse Alveolar Damage (DAD)
DAD lies on the severe end of the ALI spectrum and is the pattern typically associated with clinical acute respiratory distress syndrome (ARDS). DAD is caused by “endothelial and alveolar lining cell injury which leads to fluid and cellular exudation” (Katzenstein, Bloor, and Leibow 1976); that is, a physical disruption of multiple blood-air barriers. DAD is divided into three histological phases that generally correlate with the time from pulmonary injury, though it has been suggested in clinical studies (not histologic studies) that there may be temporal overlap between phases (Marshall et al. 2000). Rather than discrete phases, the stages of DAD may be more accurately described as a rough continuum. This is illustrated in Figure 1.
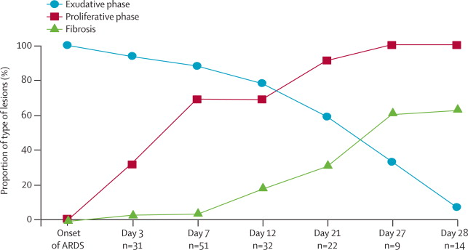
Figure 1
Time to onset and evolution of histological lesions noted over time (exudative phase, proliferative phase and fibrosis) in patients with ARDS (Thille et al. 2013).
Acute (exudative) phase: days
Classic DAD is initially characterized by intra-alveolar edema and alveolar wall thickening, typically around day two after initial injury. Hyaline membranes (the hallmark of DAD) develop around day two and typically reach peak formation at four to five days. Usually, there is only sparse inflammation unless it arises in conjunction with acute pneumonia. Vascular thrombosis and microthrombosis are frequently observed even in the absence of systemic hypercoagulability and are thought to result from local inflammation. However, when thrombus burden is significant, it can obstruct blood flow and contribute to V/Q mismatch and further exacerbate hypoxemia (Beasley 2010, Greene et al. 1987). These findings are illustrated in Figure 2A.
Subacute (organizing) phase: weeks
Approximately one week after the initial pulmonary injury, organization begins. Fibroblasts migrate into the fibrin exudates and secrete young (“loose”) collagen. Hyaline membranes slowly disappear as they become incorporated into an organizing process. Organizing fibrotic tissue begins to appear in airspaces and alveolar ducts. Reactive cytologic atypia of Type II pneumocytes and squamous metaplasia may also be present, and mitotic figures (regenerating pneumocytes) can be seen (Beasley 2010). These findings are illustrated in Figure 2B.
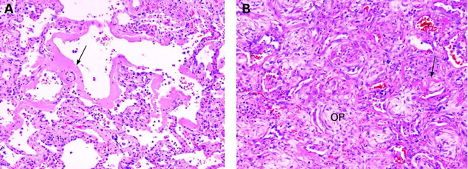
Figure 2
Acute exudative phase of DAD. (B) Subacute organizing phase of DAD. Arrows: Hyaline membranes. OP: Organizing fibrosis. (Leslie 2009).
Chronic (fibrotic) phase: months
Some cases of DAD will ultimately resolve, while others evolve to develop progressive architectural remodeling and interstitial fibrosis. In the extreme, these changes may be so severe as to resemble usual interstitial pneumonitis (UIP), the histopathological correlate of idiopathic pulmonary fibrosis (IPF).
DAD is the predominant histologic pattern associated with clinical ARDS. Some have suggested that COVID-19 is characterized histologically by injury patterns distinct from DAD (Copin et al. 2020) and have used this assertion to sub-classify COVID-19 ARDS into novel phenotypes (Gattinoni et al. 2020).
2. Acute Fibrinous and Organizing Pneumonia (AFOP)
AFOP is a pattern of lung injury characterized by fibrin aggregates within the alveolar spaces forming "fibrin balls" (Beasley et al. 2002). AFOP lacks the hyaline membranes seen in DAD, but as in DAD, fibroblasts migrate into the fibrin and secrete young collagen to organize the injury over time. This organization constitutes the organizing pneumonia (OP) component of AFOP. DAD can have features of AFOP, so it is important to exclude inadequate sampling (such as through biopsy-based autopsies) prior to making a diagnosis of AFOP. If hyaline membranes are present, then the case should be diagnosed as DAD (Beasley et al. 2002). An example of AFOP is shown in Figure 3.
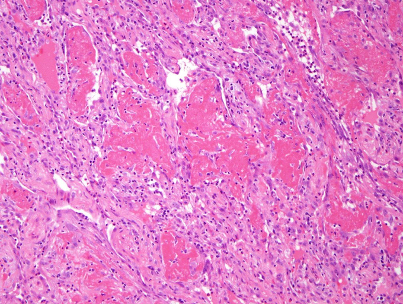
Figure 3
Acute fibrinous and organizing pneumonia (AFOP) (from the collection of Dr. Hariri).
3. Organizing Pneumonia (OP)
OP is a very common reaction pattern. It had historically been referred to as bronchiolitis obliterans organizing pneumonia (BOOP) until recently, when the pathology community decided to change the name to organizing pneumonia (OP). It was thought that the more generic descriptor of OP would avoid potential confusion with idiopathic BOOP (now cryptogenic organizing pneumonia). Clearly, the goal of increasing clarity around the term worked out really well (insert sarcasm here). To date, this pattern has not been observed in association with COVID-19 and will not be further discussed in this FLARE.
What About COVID-19?
When compared against the wealth of clinical and basic science publications on COVID-19, histological findings after human SARS-CoV-2 infection remain poorly characterized. Most of the reports rely on biopsy-based autopsies, with some reporting full autopsies, and a few reporting incidental histology from lung cancer resections in asymptomatic COVID-19 positive patients. These data are summarized in the Table below:
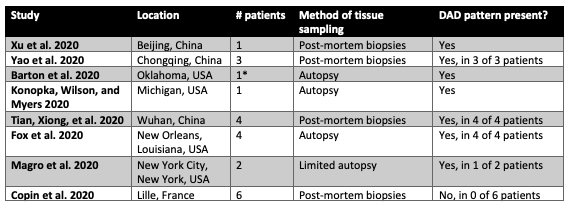
Table 1
Published and pre-print reports of lung histology in COVID-19.
*also described findings consistent with aspiration pneumonia in a patient with SARS-CoV-2 + nasopharyngeal swab but with no evidence of SARS-CoV-2 pulmonary infection.
What Are We Learning About Histological Findings in COVID-19?
Diffuse alveolar damage, acute phase: This is, by far, the predominant pattern described in the autopsy studies from COVID-19 patients. Features include prominent fibrin hyaline membranes with edema, interstitial inflammatory infiltrate, and desquamated pneumocytes with reactive pneumocyte hyperplasia. This is shown in Figure 4.
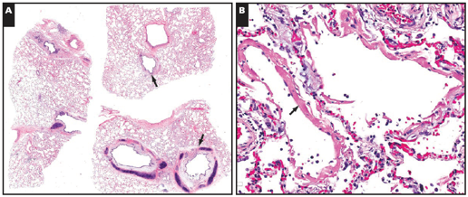
Figure 4
Diffuse alveolar damage, acute phase, in a 77 yo man with COVID-19 after 6 days of fever and chills (Barton et al. 2020).
AFOP: A few reports have described autopsy cases with regions of fibrin balls within alveolar spaces, resembling AFOP (Figure 5). However, in other complete COVID-19 pathology reports, this finding has mostly been described in a background of hyaline membranes and, therefore, is diagnosed as DAD. One report (Copin et al. 2020) has described a finding of AFOP with no hyaline membranes in six patients. However, these are biopsy-based samples described in a brief research letter with no further characterization of the patients or their illness, and it is entirely possible that hyaline membranes were not observed due to sampling method. As has been reported in other COVID-19 case reports, AFOP features can be seen in the setting of DAD (Beasley et al. 2002).
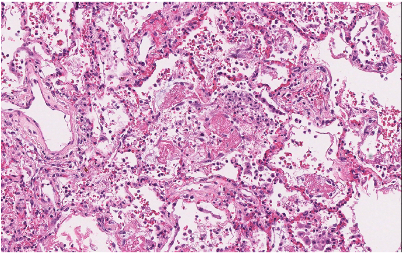
Figure 5
Diffuse alveolar damage (DAD) with regions resembling AFOP (fibrin balls within alveoli) in a 37 yo man with COVID-19, 9 days after hospitalization and 6 days after intubation (Konopka, Wilson, and Myers 2020).
Organization and Fibrosis: Nearly all published reports describe the acute phase of DAD (with little to no organization) in COVID-19 autopsies. Figure 6 and 7 below show two cases in which early (top) and more extensive organization (bottom) are present. The time course of the disease for these two patients is unclear from the respective publications.
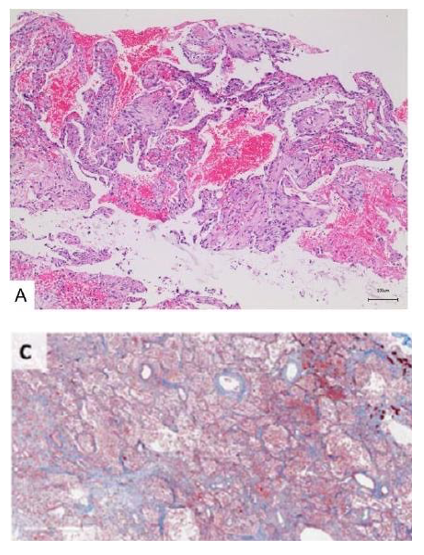
Figure 6
Early organization (A, from Yao et al. 2020) and more extensive organization of DAD in COVID-19 (C, from Barton et al. 2020).
Are There Other Histologic Features Described in COVID-19 Lung Pathology?
In short, yes. Given the sparse number of reports thus far, and the importance of considering the findings within the context of clinical history for each patient, we will not interpret the various findings individually. Representative images are shown below.
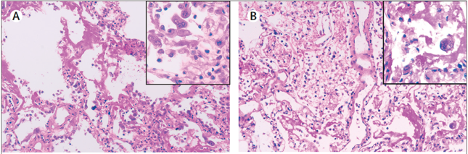
Figure 8
Multinucleated giant cells in the alveolar space (left), similar to that seen in SARS. Viral cytopathic-like changes in pneumocytes (right) (Xu et al. 2020).
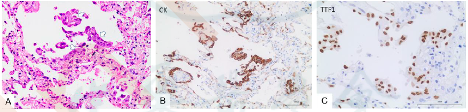
Figure 9
Pneumocyte desquamation and hyperplasia, with giant cell-like aggregates that are keratin and TTF1 positive, confirming pneumocyte origin (Xu et al. 2020).
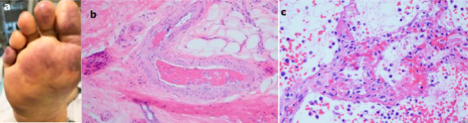
Figure 10
Multifocal microthrombotic disease involving small (and sometimes medium) sized vessels, present in the lung (C) and other organs, including the distal extremities (A and B) (Magro et al. 2020).
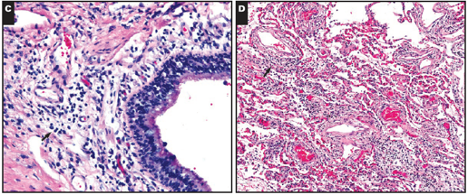
Figure 11
Chronic bronchitis with edema in a patient with DAD (Barton et al. 2020).
Several histological patterns notably have not been described in COVID-19 autopsies at this time. These include vasculitis, eosinophilic pneumonia, and granulomatous inflammation.
Histopathological Findings in Asymptomatic Patients With SARS-COV-2 Infection
A two-patient case series from China described histologic findings in asymptomatic patients who underwent lung cancer resection and were subsequently found to have COVID-19 infection at the time of surgery. As expected, the findings were not as severe as the reported cases of patients with symptomatic COVID-19 infection. The histology outside of the tumors showed edema with proteinaceous exudate, focal pneumocyte hyperplasia, and patchy chronic inflammation and multinucleated giant cells. Hyaline membranes were not identified (Tian, Hu, et al. 2020).
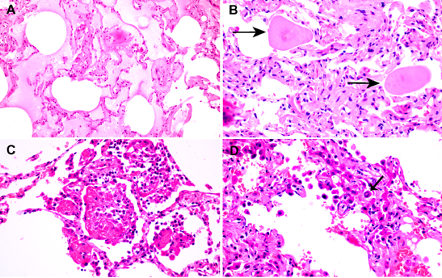
Figure 12
Early histologic findings in asymptomatic patients with COVID-19, incidentally found in lung cancer resection specimens (Tian, Hu, et al. 2020).
Not All Acute Lung Disease in 2020 Is COVID-19
In reviewing the limited number of published case reports of COVID-19 pathology, it is important to remember that distinct histologic patterns may coexist within the same patient. For example, secondary bacterial infections have been reported in patients with COVID-19 with varying incidence, reviewed in a recent FLARE on superinfections in COVID-19. One case report on COVID-19 pathology described a 42 year old man with muscular dystrophy, a 2 day history of abdominal pain, and a COVID-19 positive nasopharyngeal swab. On complete autopsy, the lung demonstrated multifocal acute bronchopneumonia with clear evidence of aspirated food material. Repeat COVID-19 testing on the lung tissue was negative. It was determined that the patient likely died with, not from, a COVID-19 infection (Barton et al. 2020). The case is a good reminder that not all pathologic observations from patients with COVID-19 are a result of COVID-19. Although COVID-19 has changed a lot about our lives and the practice of medicine, some things remain strong bedrocks on which we can always rely: aspiration will, always and forever more, remain in the differential diagnosis.
Summary
Nearly all patients in the COVID-19 autopsy reports to date have acute phase DAD, with prominent hyaline membranes and essentially no evidence of organization. Although it may be tempting to speculate about the precise origin and course of DAD in COVID-19, the data are limited and may even be subject to systematic bias due to many being biopsy-based rather than full autopsies. Furthermore, we recognize that this is a small collection of autopsy cases, and we do not have access to histological information from: 1) patients who recover from severe acute lung injury (likely DAD that moves to the organizing phase), 2) patients with milder respiratory disease with radiologic findings, and 3) patients with no symptomatic disease but with radiologic findings (likely other forms of ALI). It seems likely that these patients may have a variety of combinations of ALI patterns. However, the proportions and extent of disease in these patients remains a histologic mystery.
References:
- Barton, Lisa M., Eric J. Duval, Edana Stroberg, Subha Ghosh, and Sanjay Mukhopadhyay. 2020. “COVID-19 Autopsies, Oklahoma, USA.” American Journal of Clinical Pathology, April. https://doi.org/10.1093/ajcp/aqaa062.
- Beasley, Mary Beth. 2010. “The Pathologist’s Approach to Acute Lung Injury.” Archives of Pathology & Laboratory Medicine 134 (5): 719–27.
- Beasley, Mary Beth, Teri J. Franks, Jeffrey R. Galvin, Bernadette Gochuico, and William D. Travis. 2002. “Acute Fibrinous and Organizing Pneumonia: A Histological Pattern of Lung Injury and Possible Variant of Diffuse Alveolar Damage.” Archives of Pathology & Laboratory Medicine 126 (9): 1064–70.
- Copin, Marie-Christine, Erika Parmentier, Thibault Duburcq, Julien Poissy, Daniel Mathieu, and Lille COVID-19 ICU and Anatomopathology Group. 2020. “Time to Consider Histologic Pattern of Lung Injury to Treat Critically Ill Patients with COVID-19 Infection.” Intensive Care Medicine, April. https://doi.org/10.1007/s00134-020-06057-8.
- Fox, Sharon E., Aibek Akmatbekov, Jack L. Harbert, Guang Li, J. Quincy Brown, and Richard S. Vander Heide. 2020. “Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans.” medRxiv, April, 2020.04.06.20050575.
- Gattinoni, Luciano, Silvia Coppola, Massimo Cressoni, Mattia Busana, Sandra Rossi, and Davide Chiumello. 2020. “Covid-19 Does Not Lead to a ‘Typical’ Acute Respiratory Distress Syndrome.” American Journal of Respiratory and Critical Care Medicine, March. https://doi.org/10.1164/rccm.202003-0817LE.
- Greene, R., S. Lind, H. Jantsch, R. Wilson, K. Lynch, R. Jones, A. Carvalho, L. Reid, A. C. Waltman, and W. Zapol. 1987. “Pulmonary Vascular Obstruction in Severe ARDS: Angiographic Alterations after I.v. Fibrinolytic Therapy.” AJR. American Journal of Roentgenology 148 (3): 501–8.
- Katzenstein, A. L., C. M. Bloor, and A. A. Leibow. 1976. “Diffuse Alveolar Damage--the Role of Oxygen, Shock, and Related Factors. A Review.” The American Journal of Pathology 85 (1): 209–28.
- Konopka, Kristine E., Allecia Wilson, and Jeffrey L. Myers. 2020. “Postmortem Lung Findings in an Asthmatic with Coronavirus Disease 2019 (COVID-19).” Chest, April. https://doi.org/10.1016/j.chest.2020.04.032.
- Leslie, K. O. 2009. “My Approach to Interstitial Lung Disease Using Clinical, Radiological and Histopathological Patterns.” Journal of Clinical Pathology. https://doi.org/10.1136/jcp.2008.059782.
- Magro, Cynthia, J. Justin Mulvey, David Berlin, Gerard Nuovo, Steven Salvatore, Joanna Harp, Amelia Baxter-Stoltzfus, and Jeffrey Laurence. 2020. “Complement Associated Microvascular Injury and Thrombosis in the Pathogenesis of Severe COVID-19 Infection: A Report of Five Cases.” Translational Research: The Journal of Laboratory and Clinical Medicine, April. https://doi.org/10.1016/j.trsl.2020.04.007.
- Marshall, R. P., G. Bellingan, S. Webb, A. Puddicombe, N. Goldsack, R. J. McAnulty, and G. J. Laurent. 2000. “Fibroproliferation Occurs Early in the Acute Respiratory Distress Syndrome and Impacts on Outcome.” American Journal of Respiratory and Critical Care Medicine 162 (5): 1783–88.
- Thille, Arnaud W., Andrés Esteban, Pilar Fernández-Segoviano, José-María Rodriguez, José-Antonio Aramburu, Patricio Vargas-Errázuriz, Ana Martín-Pellicer, José A. Lorente, and Fernando Frutos-Vivar. 2013. “Chronology of Histological Lesions in Acute Respiratory Distress Syndrome with Diffuse Alveolar Damage: A Prospective Cohort Study of Clinical Autopsies.” The Lancet. Respiratory Medicine 1 (5): 395–401.
- Tian, Sufang, Weidong Hu, Li Niu, Huan Liu, Haibo Xu, and Shu-Yuan Xiao. 2020. “Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer.” Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer, February. https://doi.org/10.1016/j.jtho.2020.02.010.
- Tian, Sufang, Yong Xiong, Huan Liu, Li Niu, Jianchun Guo, Meiyan Liao, and Shu-Yuan Xiao. 2020. “Pathological Study of the 2019 Novel Coronavirus Disease (COVID-19) through Postmortem Core Biopsies.” Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc, April. https://doi.org/10.1038/s41379-020-0536-x.
- Xu, Zhe, Lei Shi, Yijin Wang, Jiyuan Zhang, Lei Huang, Chao Zhang, Shuhong Liu, et al. 2020. “Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome.” The Lancet. Respiratory Medicine, February. https://doi.org/10.1016/S2213-2600(20)30076-X.
- Yao X. H., Li T. Y., He Z. C., Ping Y. F., Liu H. W., Yu S. C., Mou H. M., et al. 2020. “[A pathological report of three COVID-19 cases by minimally invasive autopsies].” Zhonghua bing li xue za zhi Chinese journal of pathology 49 (0): E009.
View all COVID-19 updates
Learn about research in the Division of Pulmonary and Critical Care Medicine