Ventilator Liberation in COVID-19
The FLARE Four
- COVID-19 patients with respiratory failure have been reported to require long durations of mechanical ventilation, though it is not yet clear if duration will be longer than that seen with other ARDS patients
- An extensive body of literature exists on liberation from mechanical ventilation and standard approaches should be applied to COVID-19 patients
- Given the risk to providers associated with intubation, it may be appropriate to aim for a lower re-intubation rate than is typically seen in ICUs
- Some clinicians may also have a higher threshold to extubate given a desire to avoid aerosol-generating respiratory support such as HFNC and NIPPV
Many people are asking … how do we liberate patients with COVID-19 from the ventilator so that every breath you take is not assisted?
Subscribe to the latest updates from FLARE Advances in Motion
A substantial fraction of patients intubated for COVID-19 respiratory failure will recover to the point of extubation and breathe unassisted (Grasselli, Pesenti, and Cecconi 2020; Bhatraju et al. 2020; Ziehr et al. 2020). As a result, in addition to the intense interest around appropriate lung protective ventilation in these patients, there remains significant disagreement around when and how to de-escalate respiratory support in such individuals. In this FLARE, we review the evidence base for liberating patients with ARDS from mechanical ventilation and discuss a rational framework for doing so in the context of SARS-CoV-2 infection.
For How Long Are COVID-19 Patients Intubated?
A substantial percentage of critically ill COVID-19 patients require mechanical ventilation as the disease can make it harder to breathe; case series report rates of invasive mechanical ventilation in ICU patients range from 88% (Italy) (Grasselli, Pesenti, and Cecconi 2020), 71-86% (USA) (Bhatraju et al. 2020; Richardson et al. 2020; Arentz et al. 2020), 47.2% (Wuhan, China) (Wang et al. 2020), down to 15% (Wuhan, China) (Huang et al. 2020). These varying rates likely reflect inconsistent reporting, distinctive underlying population health, and variable use of non-invasive ventilation.
Evidence about how long COVID-19 patients require invasive mechanical ventilation is evolving. In a small case series (n = 21) in Seattle, 38% remained ill and mechanically ventilated at the end of the study period of 7.5 days (Arentz et al. 2020). A large Italian case series (n = 1591), where 88% of patients were intubated, reported a median ICU length of stay of 9 days (range 6-13) (Grasselli, Pesenti, and Cecconi 2020). Another small case series from Boston reported a median duration of mechanical ventilation 16 days (Ziehr et al. 2020). Perhaps the most complete data thus far comes from the Intensive Care National Audit & Research Centre (ICNARC) in the UK, who report on 4078 COVID-19 patients requiring critical care who had either been discharged or died (ICNARC 2020). Of these, 68% were intubated (for an average of 8 days), comparable to a recent large ARDS cohort reporting an average of 9 days (ICNARC 2020). A similar ARDS analysis from American patients reported an average of 6 days of mechanical ventilation (n = 457) (Kangelaris et al. 2016). Together, these early COVID-19 data suggest a duration of mechanical ventilation comparable to that previously reported for ARDS. Whether the final analysis will reveal extended intubation periods (during which the ventilator may take my (spontaneous) breath away) in COVID-19 remains to be seen.
How Do We Liberate ARDS Patients From the Ventilator?
Studies have consistently demonstrated (Hess and MacIntyre 2011) that clinicians underestimate the potential for their patients to liberate from the ventilator and may therefore needlessly prolong the process of ventilator liberation. On the other hand, extubation failure is associated with severe cardiovascular stress and increased mortality (Neil R. MacIntyre 2013). There is thus a need for data to help clinicians strike this balance and, fortunately, an extensive literature exists on methods to predict successful liberation from mechanical ventilation.
When to Switch to a Spontaneous Mode
In the setting of unresolved ARDS, spontaneous efforts and (in the case of pressure-limited modes of ventilation) associated large tidal volumes have been linked to worsened lung injury (Yoshida et al. 2017). In contrast to the relatively large literature on the effects of spontaneous breathing during early ARDS, there are relatively few studies that establish criteria for the liberalization of standard low tidal volume ventilation - either through larger tidal volumes or the switch to a spontaneous mode such as pressure support. Brochard and colleagues studied patients who had failed a 2hr trial of spontaneous breathing and compared three methods of facilitating ventilator liberation: intermittent t-piece trials, synchronized intermittent mandatory ventilation (SIMV), or pressure support ventilation (PSV) (Brochard et al. 1994). Patients on PSV were more likely to liberate from the ventilator at 21 days than patients in the other two groups. Based on this and similar data, a common approach to liberalization from low tidal ventilation is to switch to PSV when a patient is deemed ready to tolerate larger tidal volumes. Timing of the switch can be based on a number of factors, but in the absence of high quality trial evidence the decision is often based on clinical judgement. In general, patients can transition to PSV once gas exchange (as an index of recovery from lung injury) has improved as indicated by improved P:F or decreased FiO2 and PEEP (i.e. PEEP in non-obese patients ≤ 8 cm H2O, FiO2 < 50%) (N. R. MacIntyre et al. 2001). Once a patient is tolerating PSV, it is reasonable to evaluate for readiness to liberate from the ventilator.
Assessment for Extubation Readiness
Evidence for when a patient is ready to undergo a trail of readiness to liberate from the ventilator comes largely from observational trials. In 2001, several national guideline-writing organizations assembled observational reports into a single approach for discontinuing mechanical ventilation (N. R. MacIntyre et al. 2001). Consensus criteria include resolution of the underlying illness, preserved mental status, improved gas exchange and hemodynamic stability.
Periodic trials of abrupt cessation of mechanical support through a spontaneous breathing trial (SBT) remain the most effective method to liberate from mechanical ventilation (A. Esteban et al. 1995, 1999; Ely et al. 1996). An SBT is defined as a trial on minimal support (usually pressure support with 0-5 cm H2O) for a set period of time (30 min – 2 hours), typically paired with reduction in sedation (“spontaneous awakening trial” as detailed below). A protocol specifying daily SBTs has been shown to accelerate time to extubation (Figure 1): a trial of 300 patients intubated for respiratory failure randomized to either daily SBT versus usual care showed those managed on a protocol were extubated more quickly (4.5 days versus 7 days, p<0.001) (Ely et al. 1996).
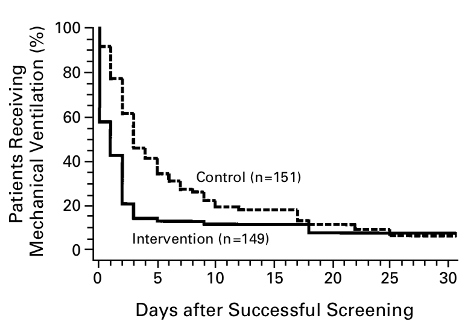
Figure 1
Kaplan-Meier Analysis of the Duration of Mechanical Ventilation after a Successful Screening Test (screening test defined by PaO2/FiO2 > 200, PEEP ≤ 5cm H2O, adequate cough, RSBI < 105 breaths/min/L, and minimal sedation/vasopressors). Intervention group then underwent protocolized, daily SBT with outcomes displayed (Ely et al. 1996).
A number of parameters have been studied for their ability to predict the success of an SBT trial. A large study at McMaster University found seven factors (some measured while receiving mechanical ventilator support and some during a short period of spontaneous breathing) that predicted success of a trial of spontaneous breathing (see table).
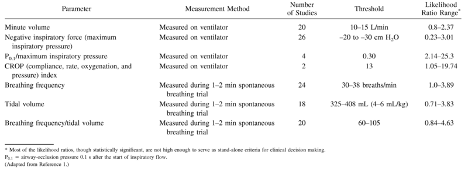
Table 1
From (Neil R. MacIntyre 2013)
An SBT is deemed successful when a patient exhibits the necessary hemodynamic, respiratory, and behavioral stability to just breathe independently and protect the airway. Criteria for assessing the tolerance of an SBT vary. Formal tools such as the rapid shallow breathing index (RSBI, the ratio of respiratory frequency to tidal volume) have been developed. In general, a RSBI “pass” is a score ≤ 105 breaths/min/L (Yang and Tobin 1991), a cutoff verified in systematic reviews with a positive likelihood ratio of 1.66 – 2.1 and negative likelihood ratio of 0.11. These data indicate high likelihood of failure with a high score (Meade et al. 2001). However, at least one randomized controlled trial has indicated that RSBI prolongs the process of ventilator discontinuation (Tanios et al. 2006).
Since reliance on single parameters such as RSBI has performed poorly in trials, guidelines typically recommend an integrated assessment that includes tidal volume, respiratory rate, work of breathing, gas exchange, cardiovascular stability and subjective assessments of patient comfort and level of distress (see table).
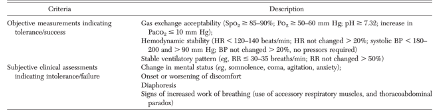
Table 2
From (Tanios et al. 2006)
For patients who fail an SBT, the specific failure mode may guide clinicians toward a different ventilator liberation strategy, reveal underlying pathology, or indicate that the patient is not ready for liberation. For example, as positive pressure is decreased, afterload on the heart increases, which may result in pulmonary edema and subsequent tachypnea and hypoxemia during an SBT. Pain, dyspnea and agitation may also present similarly, though clearly the treatment strategies are quite distinct.
Just as an assessment of a patient's respiratory performance (e.g. maintenance of tidal volumes and respiratory rate with minimal support) is essential to determine readiness for extubation, so too is an assessment of the mental status. This may include a spontaneous awakening trial (SAT), also referring to as daily interruption of sedation (DIS). The combination of daily sedation interruption (or SAT) and daily spontaneous breathing trial (SBT) has demonstrated improved patient outcomes: a randomized trial comparing daily SAT/SBT to usual care found increased ventilator-free days (14.7 days versus 11.6 days; p=0.02), reduced ICU LOS (9.1 days versus 12.9 days; p=0.01), and decreased risk of death at 1-year (HR 0.68; p=0.01) (Girard et al. 2008). These findings have been replicated in a retrospective study dedicated to the ARDS population (median duration of mechanical ventilation 14 days versus 9 days; p<0.001, median ICU LOS 18 days versus 13 days; p<0.001) (Kallet et al. 2018).
For readers interested in details about weaning sedation in COVID-19 patients, we recommend the May 2 FLARE.
What Else Should I Consider Before Extubation?
If the patient “passes” an SBT, there are other clinical characteristics that are related to extubation success that should be assessed (N. R. MacIntyre et al. 2001). These include:
Ability to cough: ineffective cough is a strong predictor for extubation failure and requirement for reintubation at 1 week (OR 5.03, 95%CI 1.8 – 14.1) (Thille et al. 2015). Heavy secretion production necessitating frequent suctioning may also increase risk of failure.
Mental status: The inability to complete four commands (open eyes, follow objects with eyes, grasp hand, and stick out tongue) is associated with increased risk of extubation failure (RR 4.3, 95% CI 1.8-10.4) (Salam et al. 2004).
Cuff leak: post-extubation stridor (April 15th FLARE) occurs in approximately 10% of patients due to vocal cord edema, secretions, or laryngeal injury, with increased rates in those with multiple intubations or prolonged intubation (Schnell et al. 2019).
Optimal timing, depending on unit schedule, staffing, and other upcoming procedures: most units prefer extubation during daytime hours, though nighttime extubation does not confer a higher likelihood of reintubation, longer length of stay, or increased mortality compared to daytime (Tischenkel et al. 2016).
Non-Invasive Respiratory Support Post-Extubation
Consensus guidelines on the management of liberation from mechanical ventilation suggest the use of non-invasive support (bi-level positive pressure, CPAP, or HFNC) post-extubation for those considered at high risk for post-extubation respiratory failure (Fan et al. 2017). Guidelines promulgated by the American Thoracic Society define high risk for post-extubation respiratory failure as age >65, chronic obstructive pulmonary disease (COPD), congestive heart failure, hypercapnia during SBT, or presence of upper airway stridor not requiring immediate re-intubation. These recommendations are based on the results of five randomized, controlled trials of non-invasive positive pressure ventilation versus usual care (supplemental oxygen) post-extubation. Four trials (Ferrer et al. 2006; Khilnani et al. 2011; Mohamed and Abdalla 2013; Nava et al. 2005) have demonstrated improved outcomes including reduced ICU length of stay, reduced respiratory distress, and reduced ICU mortality. One of the four (Khilnani et al. 2011) enrolled only patients with COPD. One study (Ferrer et al. 2006) found a reduced ICU mortality but no difference in 90-day mortality among all high-risk patients but did note a decreased 90 day mortality among the subset of patients with obstructive lung disease.
Two trials examined the use of post-extubation high flow nasal cannula (HFNC) among unselected patients post-extubation. Maggiore and colleagues (Maggiore et al. 2014) tested HFNC versus venturi mask and found a decreased re-intubation and improved patient comfort with HFNC. Hernandez and colleagues studied patients at low risk of extubation failure and compared HFNC versus conventional oxygen therapy and found a lower rate of re-intubation with HFNC.
The data above apply to the immediate post-extubation application of non-invasive support to those patients who have tolerated an SBT but are nevertheless deemed to be high risk for post-extubation respiratory failure. The data for use of NIPPV and/or HFNC in established post-extubation respiratory failure is considerably more mixed. Esteban and colleagues (Andrés Esteban et al. 2004) studied patients who developed respiratory failure within 48 hours of extubation and found an increase in time to re-intubation and increase in all cause mortality in the NIPPV group.
In sum, there is moderate quality evidence, which seems to be stronger for patients with coexistent COPD, for the protocolized use of NIPPV or HFNC post-extubation. There is considerably less support for the use of non-invasive modalities as rescue therapy in post-extubation respiratory failure - a setting in which it may delay an inevitable re-intubation and thus increase mortality.
What Are Specific Extubation Considerations in COVID-19 Patients?
Intubation and extubation are aerosol-generating procedures, given removal of positive pressure devices and associated coughing (Davies et al. 2009). Prior to COVID-19, re-intubation rates of 5-20% were generally thought to be acceptable (Neil R. MacIntyre 2013), though this figure is largely from expert opinion and little data exists. In COVID-19 patients, the following modifications have been suggested:
1) Collect additional data to support readiness for extubation
Consider an extended SBT (e.g. 30 min-2 hours) to ensure higher likelihood of successful extubation, particularly in patients who have been intubated for longer periods. The data to support this notion are both limited and heterogeneous (different protocols, amount of PSV, use of T-piece). Furthermore, they tend to include patients with shorter duration of mechanical ventilation. With this in mind, one study of patients with COPD who were intubated for longer than 15 days, the median time to SBT failure was 120 minutes (Vitacca et al. 2001). Furthermore, a prolonged SBT does not appear to demonstrate harm. In a cohort study in older critically ill patients, a prolonged SBT (8 hours) showed a reduction of failed extubation attempts, but increased SBT failures rates (Su et al. 2012). However, other cohorts showed no difference in extubation failures with SBT lasting 30 compared to 120 minutes (A. Esteban et al. 1999).
2) Consider the risk of extubation to NIPPV
Both HFNC and NIPPV are aerosol-generating procedures (Hui et al. 2019), (Hui et al. 2009), (Loh et al. 2020). While HFNC and NIPPV may be used to support recently extubated non-COVID-19 patients, the use of these modalities in COVID-19 patients should be considered against the risks of nosocomial transmission to healthcare workers. It is reasonable to consider post-extubation NIPPV in COVID-19 patients with a strong indication including obstructive lung disease or chronic hypercarbic respiratory failure (i.e. COPD), with appropriate precautions.
What else can we do to minimize time on the ventilator?
As discussed in prior FLAREs, data-driven strategies to minimize ventilator time include judicious fluid management (April 14th), optimizing PEEP and ventilator mechanics (April 2nd), and treating any secondary infection (April 27th).
Summary
We do not yet have robust data on length of mechanical ventilation needs or special considerations for COVID-19 patients. In any case, it is appropriate to apply the decades of prior research on liberation from mechanical ventilation in ICU patients. With regards to extubation, this specifically means early and daily SAT/SBTs (when appropriate), and careful consideration of the risks and benefits of HFNC/NIPPV after extubation. Ultimately, as in all ICU patients with respiratory failure, the goal of all therapy in COVID-19 is to see that patients are back to Breathin’ on their own again.
References:
- Arentz, Matt, Eric Yim, Lindy Klaff, Sharukh Lokhandwala, Francis X. Riedo, Maria Chong, and Melissa Lee. 2020. “Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State.” JAMA: The Journal of the American Medical Association, March. https://doi.org/10.1001/jama.2020.4326.
- Bhatraju, Pavan K., Bijan J. Ghassemieh, Michelle Nichols, Richard Kim, Keith R. Jerome, Arun K. Nalla, Alexander L. Greninger, et al. 2020. “Covid-19 in Critically Ill Patients in the Seattle Region - Case Series.” The New England Journal of Medicine, March. https://doi.org/10.1056/NEJMoa2004500.
- Brochard, L., A. Rauss, S. Benito, G. Conti, J. Mancebo, N. Rekik, A. Gasparetto, and F. Lemaire. 1994. “Comparison of Three Methods of Gradual Withdrawal from Ventilatory Support during Weaning from Mechanical Ventilation.” American Journal of Respiratory and Critical Care Medicine 150 (4): 896–903.
- Davies, Anna, Gail Thomson, Jimmy Walker, and Allan Bennett. 2009. “A Review of the Risks and Disease Transmission Associated with Aerosol Generating Medical Procedures.” Journal of Infection Prevention 10 (4): 122–26.
- Ely, E. W., A. M. Baker, D. P. Dunagan, H. L. Burke, A. C. Smith, P. T. Kelly, M. M. Johnson, R. W. Browder, D. L. Bowton, and E. F. Haponik. 1996. “Effect on the Duration of Mechanical Ventilation of Identifying Patients Capable of Breathing Spontaneously.” The New England Journal of Medicine 335 (25): 1864–69.
- Esteban, A., I. Alía, M. J. Tobin, A. Gil, F. Gordo, I. Vallverdú, L. Blanch, et al. 1999. “Effect of Spontaneous Breathing Trial Duration on Outcome of Attempts to Discontinue Mechanical Ventilation. Spanish Lung Failure Collaborative Group.” American Journal of Respiratory and Critical Care Medicine 159 (2): 512–18.
- Esteban, A., F. Frutos, M. J. Tobin, I. Alía, J. F. Solsona, I. Valverdú, R. Fernández, M. A. de la Cal, S. Benito, and R. Tomás. 1995. “A Comparison of Four Methods of Weaning Patients from Mechanical Ventilation. Spanish Lung Failure Collaborative Group.” The New England Journal of Medicine 332 (6): 345–50.
- Esteban, Andrés, Fernando Frutos-Vivar, Niall D. Ferguson, Yaseen Arabi, Carlos Apezteguía, Marco González, Scott K. Epstein, et al. 2004. “Noninvasive Positive-Pressure Ventilation for Respiratory Failure after Extubation.” The New England Journal of Medicine 350 (24): 2452–60.
- Fan, Eddy, Bishoy Zakhary, Andre Amaral, Jessica McCannon, Timothy D. Girard, Peter E. Morris, Jonathon D. Truwit, Kevin C. Wilson, and Carey C. Thomson. 2017. “Liberation from Mechanical Ventilation in Critically Ill Adults. An Official ATS/ACCP Clinical Practice Guideline.” Annals of the American Thoracic Society 14 (3): 441–43.
- Ferrer, Miquel, Mauricio Valencia, Josep Maria Nicolas, Oscar Bernadich, Joan Ramon Badia, and Antoni Torres. 2006. “Early Noninvasive Ventilation Averts Extubation Failure in Patients at Risk: A Randomized Trial.” American Journal of Respiratory and Critical Care Medicine 173 (2): 164–70.
- Girard, Timothy D., John P. Kress, Barry D. Fuchs, Jason W. W. Thomason, William D. Schweickert, Brenda T. Pun, Darren B. Taichman, et al. 2008. “Efficacy and Safety of a Paired Sedation and Ventilator Weaning Protocol for Mechanically Ventilated Patients in Intensive Care (Awakening and Breathing Controlled Trial): A Randomised Controlled Trial.” The Lancet 371 (9607): 126–34.
- Grasselli, Giacomo, Antonio Pesenti, and Maurizio Cecconi. 2020. “Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response.” JAMA: The Journal of the American Medical Association, March. https://doi.org/10.1001/jama.2020.4031.
- Hess, Dean R., and Neil R. MacIntyre. 2011. “Ventilator Discontinuation: Why Are We Still Weaning?” American Journal of Respiratory and Critical Care Medicine.
- Huang, Chaolin, Yeming Wang, Xingwang Li, Lili Ren, Jianping Zhao, Yi Hu, Li Zhang, et al. 2020. “Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China.” The Lancet 395 (10223): 497–506.
- Hui, David S., Benny K. Chow, Thomas Lo, Owen T. Y. Tsang, Fanny W. Ko, Susanna S. Ng, Tony Gin, and Matthew T. V. Chan. 2019. “Exhaled Air Dispersion during High-Flow Nasal Cannula Therapy versus CPAP via Different Masks.” The European Respiratory Journal: Official Journal of the European Society for Clinical Respiratory Physiology 53 (4). https://doi.org/10.1183/13993003.02339-2018.
- Hui, David S., Benny K. Chow, Susanna S. Ng, Leo C. Y. Chu, Stephen D. Hall, Tony Gin, Joseph J. Y. Sung, and Matthew T. V. Chan. 2009. “Exhaled Air Dispersion Distances during Noninvasive Ventilation via Different Respironics Face Masks.” Chest 136 (4): 998–1005.
- ICNARC. 2020. “ICNARC Report on COVID-19 in Critical Care.” Intensive Care National Audit and Research Centre.
- Kallet, Richard H., Hanjing Zhuo, Vivian Yip, Antonio Gomez, and Michael S. Lipnick. 2018. “Spontaneous Breathing Trials and Conservative Sedation Practices Reduce Mechanical Ventilation Duration in Subjects With ARDS.” Respiratory Care 63 (1): 1–10.
- Kangelaris, Kirsten Neudoerffer, Lorraine B. Ware, Chen Yu Wang, David R. Janz, Hanjing Zhuo, Michael A. Matthay, and Carolyn S. Calfee. 2016. “Timing of Intubation and Clinical Outcomes in Adults With Acute Respiratory Distress Syndrome.” Critical Care Medicine 44 (1): 120–29.
- Khilnani, G. C., A. D. Galle, V. Hadda, and S. K. Sharma. 2011. “Non-Invasive Ventilation after Extubation in Patients with Chronic Obstructive Airways Disease: A Randomised Controlled Trial.” Anaesthesia and Intensive Care 39 (2): 217–23.
- Loh, Ne-Hooi Will, Yanni Tan, Juvel Taculod, Billy Gorospe, Analine S. Teope, Jyoti Somani, and Addy Yong Hui Tan. 2020. “The Impact of High-Flow Nasal Cannula (HFNC) on Coughing Distance: Implications on Its Use during the Novel Coronavirus Disease Outbreak.” Canadian Journal of Anaesthesia = Journal Canadien D’anesthesie, March. https://doi.org/10.1007/s12630-020-01634-3.
- MacIntyre, Neil R. 2013. “The Ventilator Discontinuation Process: An Expanding Evidence Base.” Respiratory Care 58 (6): 1074–86.
- MacIntyre, N. R., D. J. Cook, E. W. Ely Jr, S. K. Epstein, J. B. Fink, J. E. Heffner, D. Hess, et al. 2001. “Evidence-Based Guidelines for Weaning and Discontinuing Ventilatory Support: A Collective Task Force Facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine.” Chest 120 (6 Suppl): 375S – 95S.
- Maggiore, Salvatore Maurizio, Francesco Antonio Idone, Rosanna Vaschetto, Rossano Festa, Andrea Cataldo, Federica Antonicelli, Luca Montini, Andrea De Gaetano, Paolo Navalesi, and Massimo Antonelli. 2014. “Nasal High-Flow versus Venturi Mask Oxygen Therapy after Extubation. Effects on Oxygenation, Comfort, and Clinical Outcome.” American Journal of Respiratory and Critical Care Medicine 190 (3): 282–88.
- Meade, M., G. Guyatt, D. Cook, L. Griffith, T. Sinuff, C. Kergl, J. Mancebo, A. Esteban, and S. Epstein. 2001. “Predicting Success in Weaning from Mechanical Ventilation.” Chest 120 (6 Suppl): 400S – 24S.
- Mohamed, Kamel Abd Elaziz, and Mohamed Hosny Abdalla. 2013. “Role of Non Invasive Ventilation in Limiting Re-Intubation after Planned Extubation.” Egyptian Journal of Chest Diseases and Tuberculosis 62 (4): 669–74.
- Nava, Stefano, Cesare Gregoretti, Francesco Fanfulla, Enzo Squadrone, Mario Grassi, Annalisa Carlucci, Fabio Beltrame, and Paolo Navalesi. 2005. “Noninvasive Ventilation to Prevent Respiratory Failure after Extubation in High-Risk Patients.” Critical Care Medicine 33 (11): 2465–70.
- Richardson, Safiya, Jamie S. Hirsch, Mangala Narasimhan, James M. Crawford, Thomas McGinn, Karina W. Davidson, and the Northwell COVID-19 Research Consortium, et al. 2020. “Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area.” JAMA: The Journal of the American Medical Association, April. https://doi.org/10.1001/jama.2020.6775.
- Salam, Adil, Lisa Tilluckdharry, Yaw Amoateng-Adjepong, and Constantine A. Manthous. 2004. “Neurologic Status, Cough, Secretions and Extubation Outcomes.” Intensive Care Medicine 30 (7): 1334–39.
- Schnell, David, Benjamin Planquette, Asaël Berger, Sybille Merceron, Julien Mayaux, Lucas Strasbach, Stéphane Legriel, Sandrine Valade, Michael Darmon, and Ferhat Meziani. 2019. “Cuff Leak Test for the Diagnosis of Post-Extubation Stridor: A Multicenter Evaluation Study.” Journal of Intensive Care Medicine 34 (5): 391–96.
- Su, Kang-Cheng, Cheng-Chien Tsai, Kun-Ta Chou, Chong-Chen Lu, Yung-Yang Liu, Chun-Sheng Chen, Yu-Chung Wu, Yu-Chin Lee, and Diahn-Warng Perng. 2012. “Spontaneous Breathing Trial Needs to Be Prolonged in Critically Ill and Older Patients Requiring Mechanical Ventilation.” Journal of Critical Care 27 (3): 324.e1–7.
- Tanios, Maged A., Michael L. Nevins, Katherine P. Hendra, Pierre Cardinal, Jill E. Allan, Elena N. Naumova, and Scott K. Epstein. 2006. “A Randomized, Controlled Trial of the Role of Weaning Predictors in Clinical Decision Making.” Critical Care Medicine 34 (10): 2530–35.
- Thille, Arnaud W., Florence Boissier, Hassen Ben Ghezala, Keyvan Razazi, Armand Mekontso-Dessap, and Christian Brun-Buisson. 2015. “Risk Factors for and Prediction by Caregivers of Extubation Failure in ICU Patients: A Prospective Study.” Critical Care Medicine 43 (3): 613–20.
- Tischenkel, Bryan R., Michelle N. Gong, Ariel L. Shiloh, Vincent C. Pittignano, Yonatan G. Keschner, Jesse A. Glueck, Hillel W. Cohen, and Lewis A. Eisen. 2016. “Daytime Versus Nighttime Extubations: A Comparison of Reintubation, Length of Stay, and Mortality.” Journal of Intensive Care Medicine 31 (2): 118–26.
- Vitacca, M., A. Vianello, D. Colombo, E. Clini, R. Porta, L. Bianchi, G. Arcaro, et al. 2001. “Comparison of Two Methods for Weaning Patients with Chronic Obstructive Pulmonary Disease Requiring Mechanical Ventilation for More than 15 Days.” American Journal of Respiratory and Critical Care Medicine 164 (2): 225–30.
- Wang, Dawei, Bo Hu, Chang Hu, Fangfang Zhu, Xing Liu, Jing Zhang, Binbin Wang, et al. 2020. “Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China.” JAMA: The Journal of the American Medical Association, February. https://doi.org/10.1001/jama.2020.1585.
- Yang, K. L., and M. J. Tobin. 1991. “A Prospective Study of Indexes Predicting the Outcome of Trials of Weaning from Mechanical Ventilation.” The New England Journal of Medicine 324 (21): 1445–50.
- Yoshida, Takeshi, Yuji Fujino, Marcelo B. P. Amato, and Brian P. Kavanagh. 2017. “Fifty Years of Research in ARDS. Spontaneous Breathing during Mechanical Ventilation. Risks, Mechanisms, and Management.” American Journal of Respiratory and Critical Care Medicine 195 (8): 985–92.
- Ziehr, David R., Jehan Alladina, Camille R. Petri, Jason H. Maley, Ari Moskowitz, Benjamin D. Medoff, Kathryn A. Hibbert, B. Taylor Thompson, and C. Corey Hardin. 2020. “Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study.” American Journal of Respiratory and Critical Care Medicine, April. https://doi.org/10.1164/rccm.202004-1163LE.
View all COVID-19 updates
Learn more about research in the Division of Pulmonary and Critical Care Medicine