LFTs in COVID-19
The FLARE Four
- Elevated liver biochemistries (LFTs) are common in hospitalized patients with COVID-19, and higher in sicker patients; however, mortality in COVID-19 does not appear to be driven, even to a small degree, by liver failure
- If there are “classic” COVID-19 LFTs, they consist of a hepatocellular pattern of injury with transaminases 1 to 5 times the upper limit of normal and AST > ALT
- While non-hepatic sources can increase the level of some tests (AST, for example), elevated LFTs in most cases of COVID-19 probably do represent a primary liver injury
- Primary liver injury, however, may result from a complicated mixture of underlying liver disease, COVID-19-related liver injury, drug-induced liver injury, ischemia, cholestasis of sepsis, and other forms of liver injury not specific to COVID-19
Many people are saying...what's up with the LFTs in COVID-19?
Subscribe to the latest updates from FLARE Advances in Motion
Introduction
Elevated liver biochemistries (often called LFTs and generally encompassing AST, ALT, alkaline phosphatase, and total and direct bilirubin, +/- GGT) are common in patients hospitalized with COVID-19. Occasionally these elevations reach a threshold that triggers clinical concern. In tonight’s FLARE, we describe what is known about LFTs in COVID-19 as well as the clinical implications of elevated LFTs in this disease.
Elevated LFTs Are Common in COVID-19
Let’s review a few caveats regarding the available literature. First, most data about LFTs in COVID-19 come from large cohort studies in China. The location is relevant because the incidence of many liver diseases differs between China, the United States, and other parts of the world. Second, most of these large studies have reported only admission LFTs, with few reported LFTs during the course of admission. Third, which tests are included as “LFTs” also varies between studies (e.g. gamma-glutamyltransferase or albumin). Fourth, different labs use different reference ranges, especially for transaminases. Finally, clinicians should be aware of the difference between simply elevated LFTs and those abnormal enough to be deemed “liver injury”. With this in mind, let us review the data.
In nine Chinese cohort studies, 15-53% of patients had abnormal LFTs on admission, although most frequently this abnormality was mild - just above the upper limit of normal. Prior baseline information was not provided, so it is unknown whether these slight abnormalities represented an acute phenomenon (N. Chen et al. 2020; J. Chen et al. 2020; Guan et al. 2020; Huang et al. 2020; Wang et al. 2020; Yang et al. 2020; Fan et al. 2020; Cai, Huang, Yu, et al. 2020; Zhou et al. 2020).
During hospitalization, approximately 75% of patients in two Chinese cohorts developed LFTs above the upper limit of normal (ULN) (Fan et al. 2020; Cai, Huang, Yu, et al. 2020). In a cohort of 417 patients and another with 5771 patients admitted with COVID-19, 11.6% and 6.2% developed LFTs over 3 times ULN (Cai, Huang, Yu, et al. 2020; Lei et al. 2020).
In terms of data from American centers, we are aware of 6 case series reporting LFTs. In the largest US cohort to date, published by Richarson and colleagues, 58.4% of patients had AST greater than the ULN, and 39% had ALT greater than the ULN on presentation (Richardson et al. 2020).
In a study of 393 patients from Weill Cornell Medical Center and its affiliated hospital, 32% of COVID-19 patients had an elevated ALT and 46.5% had an elevated AST (this increased to 37.5% and 63% of mechanically ventilated patients) (Goyal et al. 2020).
In a study of 377 inpatients from Kaiser Permanente health system in Northern California, median values of AST, ALT and total bilirubin were normal at admission, including for 113 patients who were hospitalized in the ICU (Myers et al. 2020). In a cohort study of 250 American patients with COVID-19 and no chronic liver disease, 67.5% developed elevated AST and 50.6% developed elevated ALT (Singh and Khan 2020). Of note, this study did not include information on the severity of COVID-19 disease.
Focusing on critically ill patients, in two small studies of ICU patients in Washington state, 41% and 32% of patients had elevated AST and ALT on arrival (Bhatraju et al. 2020), and 14% developed transaminases 3 times the ULN (Arentz et al. 2020).
Combining this information, abnormal LFTs on admission are not uncommon in patients with COVID-19, and many patients also have abnormal LFTs at some point during hospitalization.
Elevated LFTs have been seen with other SARS viruses and pandemic influenza. In SARS, mean ALT rose from 60 IU/L on day 1 to 90 IU/L on day 7 (Lee et al. 2003) and in the related MERS-CoV virus, AST and ALT were elevated on admission in 11%, and 15% patients in a case series of 47 patients (Assiri et al. 2013). In H1N1 pandemic influenza, the mean AST was 49 U/L and ALT was 46 U/L (Papic et al. 2012). This is notable as transaminitis is not typically seen in seasonal flu (Papic et al. 2012).
Abnormal LFTS Are Associated With Worse Outcomes in COVID-19
Admission LFTs are higher in patients with COVID-19 who do poorly, in nearly every cohort and with almost any definition of poor outcomes. However, the degree of LFT elevation is often mild and may even fall within the range of normal (see figure below).
In 10 Chinese cohort studies, abnormal LFTs were linked to outcomes (J. Chen et al. 2020; Cai, Huang, Yu, et al. 2020; Deng et al. 2020; Guan et al. 2020; Huang et al. 2020; Wang et al. 2020; Zhou et al. 2020; Fan et al. 2020; Lei et al. 2020; T. Chen et al. 2020). In most of these cohorts, admission AST was associated with worse outcomes, including ICU admission, longer hospital length of stay, and death. In three cohorts, ALT was associated with worse outcomes (Wang et al. 2020; Zhou et al. 2020; Lei et al. 2020).
While the association with outcome is consistent, the level of elevation has generally been mild. For example, in a cohort of 1099 patients, AST > 40 U/L was the cut-off for higher-risk (Guan et al. 2020). In the largest cohort of 5771 patients, an adjusted regression found AST 40-120 U/L and > 120 U/L associated with mortality (40-120 U/L: HR 4.81 [3.83, 6.86]; >120 U/L: 14.87 [9.64, 22.93]) (Lei et al. 2020).
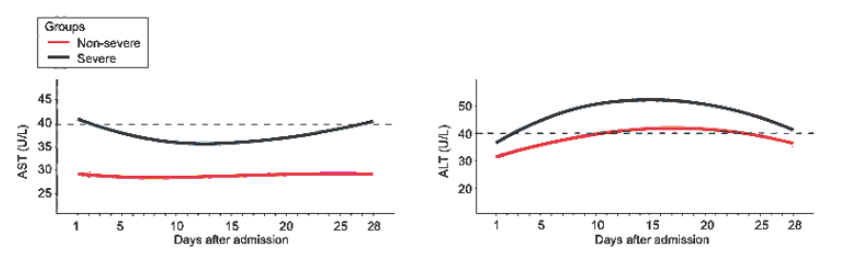
Figure 1
Kernel density estimates using Gaussian kernels to display an overlay of ALT and AST distributions by disease severity (Lei et al. 2020)
Despite the high incidence of abnormal LFTs in COVID-19, there have been no reports of liver failure or liver dysfunction leading to death attributed directly to SARS-CoV-2 infection; this is not surprising as the liver abnormalities have, on average, been mild.
There have been two published cases of severe liver injury in patients with COVID-19. The first was a patient whose enzymes reached ALT 7590 U/L and AST 1445 U/L, but we have no description of the clinical context, presence of overt liver failure, or the outcomes of that patient (N. Chen et al. 2020). The second case was that of severe hepatitis in a patient with HIV, who presented with AST 1230 U/L and ALT 697 U/L. These LFT abnormalities could not be attributed to anything other than COVID-19, and resolved spontaneously (Wander, Epstein, and Bernstein 2020).
What Are the Different Patterns of Liver Injury in COVID-19?
Once again, we are making inferences from limited data. Most published data are from the point of admission, but we know that LFTs can change over the course of hospitalization.
On admission, it appears that transaminases are far more likely to be elevated than alkaline phosphatase or total bilirubin (Guan et al. 2020; Cai, Huang, Yu, et al. 2020; Cai, Huang, Ou, et al. 2020). Furthermore, AST is often greater than ALT on admission, especially in those with elevated LFTs (Liu et al. 2020). These elevations, though, are generally mild.
In a cohort of 417 COVID-19 patients in China, 78% of whom had mild disease, 90 met criteria for liver injury (AST or ALT > 3× ULN, ALP, GGT, and/or TBIL over 2× ULN) during hospitalization. In that group, ALT was actually more likely to be above 3 times the ULN (37%) than AST (20%). In that same group, alkaline phosphatase and total bilirubin surpassed 3 times the ULN in only 9 and 1 patient respectively (Cai, Huang, Yu, et al. 2020). However, this group also included patients with severe COVID-19, including 10 with multi-organ failure, who were much more likely to receive hepatotoxic medications (e.g. antibiotics, NSAIDs, Chinese herbal medications, and interferon). Furthermore, in a multivariate logistic regression, use of lopinavir/ritonavir was associated with a significantly increased risk of liver injury (OR 4.44; 95% CI 1.50–13.17, p < 0.01), which may have accounted, in part, for liver injury.
In the largest cohort study to date to investigate LFTs in COVID-19, 5771 adult patients were found to have AST elevation on admission and throughout the course of their hospitalizations (Lei et al. 2020). ALT rose over the course of hospitalization and peaked, above the ULN, within 10 to 15 days of admission in the severe disease group. Alkaline phosphatase rose during hospitalization but generally stayed within the normal range. Fluctuations in total bilirubin were mild.
It therefore appears that the pattern of COVID-19-associated liver injury is hepatocellular, with AST greater than ALT. There are, of course, exceptions.
What Causes Elevated LFTs in COVID-19?
We don’t know yet, but many hypotheses have been put forward, most based on only preliminary data. There may be a viral-mediated effect as COVID-19 has been associated with higher LFTs than those admitted at the same time with non-COVID pneumonia (Zhao et al. 2020). The AST predominance may also be a clue, and should lead us to look at other conditions including those with prominent AST elevations.
- Muscle injury: Muscle contains AST, and case reports have described rhabdomyolysis with elevated AST (Jin and Tong 2020) in COVID-19
Hepatic steatosis: the single published liver biopsy of a patient with COVID-19 showed microvesicular steatosis and mild lobular and portal activity (Xu et al. 2020). AST predominance is seen in alcohol-related liver injury due in part to hepatic steatosis - Micro-thrombotic disease: Abnormal coagulation markers have been seen in COVID-19 and discussed in a recent FLARE. Ischemia is a known cause of AST-predominant LFT elevation (Tapper, Sengupta, and Bonder 2015), and micro-thrombotic events could be contributing to liver injury in COVID-19
- Direct viral infection: ACE2, the major receptor for SARS-CoV-2 infectivity, is expressed in the liver (Hamming et al. 2004)
Better established than the above hypotheses is the association between LFTs and hepatotoxic drugs. Many patients with COVID-19 are being simultaneously initiated on several potentially hepatotoxic therapies (see table). While approximately half of patients present to the hospital with abnormal LFTs, new LFT elevations or rising LFTs may be attributable to drug-induced liver injury. In the large Chinese series by Lei and colleagues (Lei et al. 2020), peak levels of liver function enzymes were significantly associated with use of antibiotics, antivirals and antifungals.
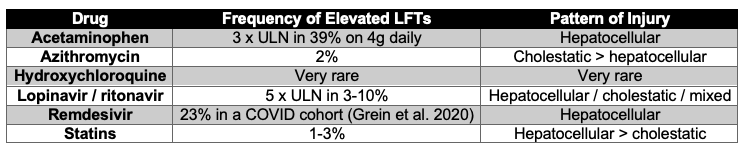
Table 1
Data adapted from livertox. NIH.gov unless specified.
Conclusions
Elevated LFTs are common in COVID-19, and associated with more severe disease in observational studies. There are numerous hypotheses about why the LFTs are elevated, but we do not yet know the precise mechanism by which COVID-19 influences the liver. Moreover, abnormal liver biochemistries may be secondary to therapeutic complications rather than sequelae of viral infection. It appears that severe liver injury from COVID-19 is rare, and that liver failure is not driving mortality.
References:
- Arentz, Matt, Eric Yim, Lindy Klaff, Sharukh Lokhandwala, Francis X. Riedo, Maria Chong, and Melissa Lee. 2020. “Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State.” JAMA: The Journal of the American Medical Association, March. https://doi.org/10.1001/jama.2020.4326.
- Assiri, Abdullah, Jaffar A. Al-Tawfiq, Abdullah A. Al-Rabeeah, Fahad A. Al-Rabiah, Sami Al-Hajjar, Ali Al-Barrak, Hesham Flemban, et al. 2013. “Epidemiological, Demographic, and Clinical Characteristics of 47 Cases of Middle East Respiratory Syndrome Coronavirus Disease from Saudi Arabia: A Descriptive Study.” The Lancet Infectious Diseases 13 (9): 752–61.
- Bhatraju, Pavan K., Bijan J. Ghassemieh, Michelle Nichols, Richard Kim, Keith R. Jerome, Arun K. Nalla, Alexander L. Greninger, et al. 2020. “Covid-19 in Critically Ill Patients in the Seattle Region - Case Series.” The New England Journal of Medicine, March. https://doi.org/10.1056/NEJMoa2004500.
- Cai, Qingxian, Deliang Huang, Pengcheng Ou, Hong Yu, Zhibin Zhu, Zhang Xia, Yinan Su, et al. 2020. “COVID-19 in a Designated Infectious Diseases Hospital Outside Hubei Province, China.” Allergy, April. https://doi.org/10.1111/all.14309.
- Cai, Qingxian, Deliang Huang, Hong Yu, Zhibin Zhu, Zhang Xia, Yinan Su, Zhiwei Li, et al. 2020. “COVID-19: Abnormal Liver Function Tests.” Journal of Hepatology, April. https://doi.org/10.1016/j.jhep.2020.04.006.
- Chen, Jun, Tangkai Qi, Li Liu, Yun Ling, Zhiping Qian, Tao Li, Feng Li, et al. 2020. “Clinical Progression of Patients with COVID-19 in Shanghai, China.” The Journal of Infection 80 (5): e1–6.
- Chen, Nanshan, Min Zhou, Xuan Dong, Jieming Qu, Fengyun Gong, Yang Han, Yang Qiu, et al. 2020. “Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study.” The Lancet 395 (10223): 507–13.
- Chen, Tao, Di Wu, Huilong Chen, Weiming Yan, Danlei Yang, Guang Chen, Ke Ma, et al. 2020. “Clinical Characteristics of 113 Deceased Patients with Coronavirus Disease 2019: Retrospective Study.” BMJ 368 (March): m1091.
- Deng, Yan, Wei Liu, Kui Liu, Yuan-Yuan Fang, Jin Shang, Ling Zhou, Ke Wang, et al. 2020. “Clinical Characteristics of Fatal and Recovered Cases of Coronavirus Disease 2019 (COVID-19) in Wuhan, China: A Retrospective Study.” Chinese Medical Journal, March. https://doi.org/10.1097/CM9.0000000000000824.
- Fan, Zhenyu, Liping Chen, Jun Li, Xin Cheng, Jingmao Yang, Cheng Tian, Yajun Zhang, Shaoping Huang, Zhanju Liu, and Jilin Cheng. 2020. “Clinical Features of COVID-19-Related Liver Damage.” Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association, April. https://doi.org/10.1016/j.cgh.2020.04.002.
- Goyal, Parag, Justin J. Choi, Laura C. Pinheiro, Edward J. Schenck, Ruijun Chen, Assem Jabri, Michael J. Satlin, et al. 2020. “Clinical Characteristics of Covid-19 in New York City.” The New England Journal of Medicine, April. https://doi.org/10.1056/NEJMc2010419.
- Grein, Jonathan, Norio Ohmagari, Daniel Shin, George Diaz, Erika Asperges, Antonella Castagna, Torsten Feldt, et al. 2020. “Compassionate Use of Remdesivir for Patients with Severe Covid-19.” The New England Journal of Medicine, April. https://doi.org/10.1056/NEJMoa2007016.
- Guan, Wei-Jie, Zheng-Yi Ni, Yu Hu, Wen-Hua Liang, Chun-Quan Ou, Jian-Xing He, Lei Liu, et al. 2020. “Clinical Characteristics of Coronavirus Disease 2019 in China.” The New England Journal of Medicine, February. https://doi.org/10.1056/NEJMoa2002032.
- Hamming, I., W. Timens, M. L. C. Bulthuis, A. T. Lely, G. J. Navis, and H. van Goor. 2004. “Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis.” The Journal of Pathology 203 (2): 631–37.
- Huang, Chaolin, Yeming Wang, Xingwang Li, Lili Ren, Jianping Zhao, Yi Hu, Li Zhang, et al. 2020. “Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China.” The Lancet 395 (10223): 497–506.
- Jin, Min, and Qiaoxia Tong. 2020. “Rhabdomyolysis as Potential Late Complication Associated with COVID-19.” Emerging Infectious Diseases 26 (7). https://doi.org/10.3201/eid2607.200445.
- Lee, Nelson, David Hui, Alan Wu, Paul Chan, Peter Cameron, Gavin M. Joynt, Anil Ahuja, et al. 2003. “A Major Outbreak of Severe Acute Respiratory Syndrome in Hong Kong.” The New England Journal of Medicine 348 (20): 1986–94.
- Lei, Fang, Ye-Mao Liu, Feng Zhou, Juan-Juan Qin, Peng Zhang, Lihua Zhu, Xiao-Jing Zhang, et al. 2020. “Longitudinal Association between Markers of Liver Injury and Mortality in COVID-19 in China.” Hepatology , May. https://doi.org/10.1002/hep.31301.
- Liu, Wei, Zhao-Wu Tao, Wang Lei, Yuan Ming-Li, Liu Kui, Zhou Ling, Wei Shuang, et al. 2020. “Analysis of Factors Associated with Disease Outcomes in Hospitalized Patients with 2019 Novel Coronavirus Disease.” Chinese Medical Journal, February. https://doi.org/10.1097/CM9.0000000000000775.
- Myers, Laura C., Stephen M. Parodi, Gabriel J. Escobar, and Vincent X. Liu. 2020. “Characteristics of Hospitalized Adults With COVID-19 in an Integrated Health Care System in California.” JAMA: The Journal of the American Medical Association, April. https://doi.org/10.1001/jama.2020.7202.
- Papic, Neven, Ana Pangercic, Martina Vargovic, Bruno Barsic, Adriana Vince, and Ilija Kuzman. 2012. “Liver Involvement during Influenza Infection: Perspective on the 2009 Influenza Pandemic.” Influenza and Other Respiratory Viruses 6 (3): e2–5.
- Richardson, Safiya, Jamie S. Hirsch, Mangala Narasimhan, James M. Crawford, Thomas McGinn, Karina W. Davidson, and the Northwell COVID-19 Research Consortium, et al. 2020. “Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area.” JAMA: The Journal of the American Medical Association, April. https://doi.org/10.1001/jama.2020.6775.
- Singh, Shailendra, and Ahmad Khan. 2020. “Clinical Characteristics and Outcomes of COVID-19 Among Patients with Pre-Existing Liver Disease in United States: A Multi-Center Research Network Study.” Gastroenterology, May. https://doi.org/10.1053/j.gastro.2020.04.064.
- Tapper, Elliot B., Neil Sengupta, and Alan Bonder. 2015. “The Incidence and Outcomes of Ischemic Hepatitis: A Systematic Review with Meta-Analysis.” The American Journal of Medicine 128 (12): 1314–21.
- Wander, Praneet, Marcia Epstein, and David Bernstein. 2020. “COVID-19 Presenting as Acute Hepatitis.” The American Journal of Gastroenterology, April. https://doi.org/10.14309/ajg.0000000000000660.
- Wang, Dawei, Bo Hu, Chang Hu, Fangfang Zhu, Xing Liu, Jing Zhang, Binbin Wang, et al. 2020. “Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China.” JAMA: The Journal of the American Medical Association, February. https://doi.org/10.1001/jama.2020.1585.
- Xu, Zhe, Lei Shi, Yijin Wang, Jiyuan Zhang, Lei Huang, Chao Zhang, Shuhong Liu, et al. 2020. “Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome.” The Lancet. Respiratory Medicine, February. https://doi.org/10.1016/S2213-2600(20)30076-X.
- Yang, Xiaobo, Yuan Yu, Jiqian Xu, Huaqing Shu, Jia ’an Xia, Hong Liu, Yongran Wu, et al. 2020. “Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study.” The Lancet. Respiratory Medicine, February. https://doi.org/10.1016/S2213-2600(20)30079-5.
- Zhao, Dahai, Feifei Yao, Lijie Wang, Ling Zheng, Yongjun Gao, Jun Ye, Feng Guo, Hui Zhao, and Rongbao Gao. 2020. “A Comparative Study on the Clinical Features of COVID-19 Pneumonia to Other Pneumonias.” Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, March. https://doi.org/10.1093/cid/ciaa247.
- Zhou, Fei, Ting Yu, Ronghui Du, Guohui Fan, Ying Liu, Zhibo Liu, Jie Xiang, et al. 2020. “Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study.” The Lancet 395 (10229): 1054–62.
Learn more about research in the Division of Pulmonary and Critical Care Medicine
View all COVID-19 updates