Considerations for Pregnant Women and Their Neonates
The FLARE Four
- COVID-19 may be associated with critical illness, including during pregnancy
- Treatment of critical illness in pregnancy may require consideration of the timing of delivery. This decision must balance the effects of delivery on the neonate as well as on the mother
- COVID-19, by itself, is currently not an indication for cesarean delivery. Instead, a decision to proceed with cesarean delivery should be made based on standard obstetrical indications
- The desire to prevent peripartum transmission of SARS-CoV-2 complicates decisions around breastfeeding and contact between mother and infant
Many people are asking...how does SARS-CoV-2 change peri- and postpartum care?
Subscribe to the latest updates from FLARE Advances in Motion
What Do We Know About Severe COVID-19 and Delivery?
Management of critically ill pregnant women is complex, due both to the increased risk of spontaneous preterm labor (Auger et al., 2011) and concerns regarding cardiovascular and respiratory physiologic changes which make pregnant women more susceptible to rapid decompensation, thereby complicating resuscitation (see Table). Depending on maternal status, response to supportive care, and gestational age, critical illness in pregnancy may require a decision about delivery. The decision to deliver must weigh risks and benefits to the neonate as well to the mother and is informed by the myriad physiological changes which occur in normal pregnancy. Moreover, delivery itself often precipitates significant hemodynamic changes (Ouzounian and Elkayam, 2012) owing to autotransfusion (~500cc), catecholamine surge, and release of inflammatory substances.
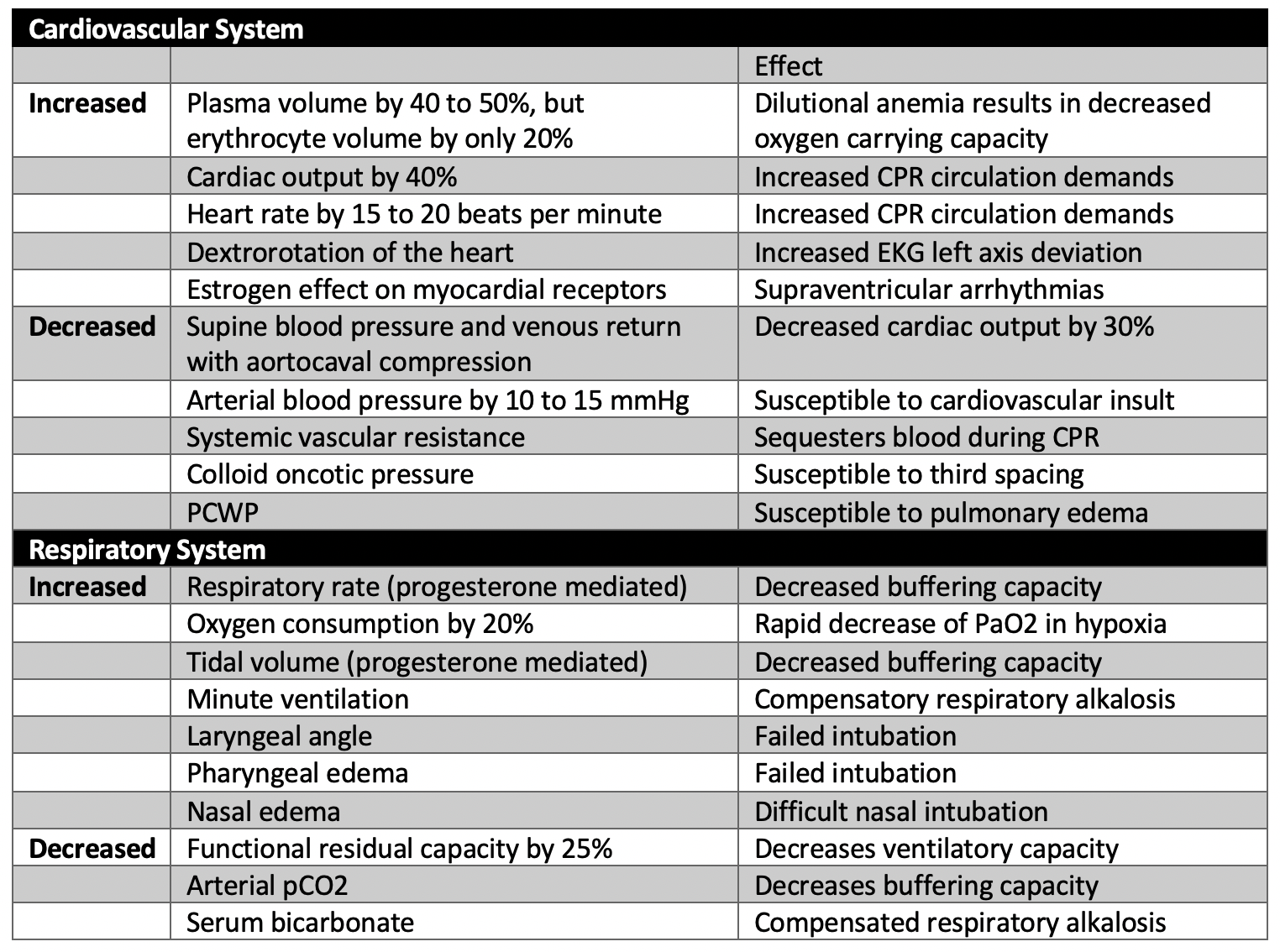
Table
Physiologic changes of pregnancy that affect resuscitation and critical illness. For more information see: ACOG practice bulletin, Critical Care in Pregnancy (2019).
Data on delivery in the setting of COVID-19 are sparse. In a recently published cohort of 64 severely and critically ill pregnant women with COVID-19 from 12 institutions, mean gestational age at onset was ~30 weeks and half of women were delivered during their hospitalization (Pierce-Williams et al., 2020), most commonly for maternal/obstetric indications. 34% of severely ill patients and 85% of critically ill patients were delivered, of which 88% were preterm - though the average gestational age at delivery even for those who were critically ill was 32.4 weeks. Median time between symptom onset and delivery was 10 days, consistent with known disease kinetics in COVID-19. Reassuringly, there were no reports of perinatal or maternal death in this cohort.
Unlike some causes of peripartum critical illness (e.g. preeclampsia), case reports suggest that delivery does not universally improve outcome or avoid complications in COVID-19. Breslin and colleagues reported on two SARS-CoV-2 infected women who were asymptomatic when admitted for routine labor induction but ultimately became critically ill in the postpartum period (Breslin et al., 2020). One was intubated due to respiratory distress during cesarean delivery and another developed respiratory distress and severe hypertension more than a day after delivery. Schnettler and colleagues describe a case in which a 32 week delivery was indicated due to non-reassuring fetal status in an intubated patient; delivery was uncomplicated but the patient remained intubated at the conclusion of the study, more than a week later (Schnettler et al., 2020). In a case series of 9 critically ill women from Iran, there were 7 fatalities. 5 of the 7 fatalities were among women who delivered 24 or more hours prior to decompensating (Hantoushzadeh et al., 2020), and a recent case report also describes a patient who presented with mild symptoms but subsequently died within 36 hours of admission, in spite of delivery of a healthy neonate in the interim (Vallejo and Ilagan, 2020).
Taken together, this evidence indicates that timing of delivery should be determined by maternal disease course and trajectory and maternal safety, as well as the usual obstetric indications. If there are obstetric indications for early delivery, delivery should not be delayed, and if preterm labor develops, attempts to delay delivery as would be standard in the absence of infection should be deferred. If infection of COVID-19 is not improved by ‘treatment’ (time, medications, other supportive measures), delivery may be considered even in the absence of obstetric indications. Though there is controversy surrounding the use of corticosteroids in COVID-19 patients, a short course of antenatal corticosteroids for fetal benefit may be considered if preterm delivery prior to 34 weeks is anticipated (SMFM, 2020).
Early Reports Describe a High Cesarean Section Rate. Is There a Benefit for Cesarean Delivery in Comparison to Vaginal Delivery?
Early reports from China described a very high rate of cesarean section among women with SARS-CoV-2 infection: 92% in a meta-analysis of SARS, MERS and SARS-CoV-2 infections, and 93% in a case series of 118 women (Chen et al., 2020b; Di Mascio et al., 2020). However, these reports consisted largely of pregnant women presenting with pneumonia (92% and 79% respectively) and do not clearly define the indications for cesarean delivery leaving uncertainty as to whether these operative deliveries were performed due to fetal, maternal, or institutional indications. Additionally, these reports did not describe mode of delivery among women with more mild to moderate disease.
Given the current practice of universal COVID-19 testing for all pregnant women admitted to labor and delivery at many centers around the country, we are recognizing that the large majority of pregnant women with COVID-19 are asymptomatic, presymptomatic, or mildly symptomatic (Sutton et al., 2020) and may therefore be expected to have uncomplicated deliveries. In a series of 43 pregnant test-confirmed COVID-19-positive women admitted for labor in two NY hospitals, 86% of women had mild disease (Breslin et al., 2020). 18 of the 43 women delivered, including four symptomatic women and 14 asymptomatic women. In this more representative cohort of the US experience, 8 of 18 women (44.4%) were delivered via cesarean section. The indications were distributed between non-reassuring fetal heart rate, arrest of progress in labor, and prior cesarean section. Maternal COVID-19 status was not reported to drive decisions about cesarean delivery. 10 of 18 women (55.5%) had uncomplicated normal vaginal deliveries. These data, both more contemporary and generalizable, suggest that vaginal delivery is a viable option for the majority of women. Therefore, no clear COVID-19 specific indication for cesarean delivery exists and decisions on delivery mode should be made based on standard obstetrical indications.
Peripartum Transmission of Sars-Cov-2 From Mother to Baby Is a Concern. What Could This Mean for Mother-Baby Care in the Hospital and After Discharge?
Delivery and Potential for Transmission
To date, there is no molecular evidence for definitive vertical transmission across the placenta, though rigorous attempts to answer this question have yet to be performed. There are several case series and reports of newborns testing positive prior to discharge, but it is unclear if vertical, perinatal, or postnatal transmission occurs in these cases (see April 20th FLARE). One study showed that virus can be detected in both the blood and stool of infected women, which infants are often exposed to during the delivery process (Chen et al., 2020a). Another case report identified SARS-CoV-2 virus in the placenta (Baud et al., 2020).
The American Academy of Pediatrics currently recommends routine testing of infants born to SARS-CoV-2 positive mothers at 24hrs and then 48hrs of life, if still inpatient.
Separation of Mothers and Infants
Currently the AAP and CDC recognize that separation of mothers and newborns is the primary way to ensure the infant is protected from infection. However, as mentioned above, case reports exist of infants acquiring the virus despite full separation. Separation is theoretically most important in cases where the mother is experiencing significant symptoms. To date, there is no available data regarding molecular testing on neonates born to asymptomatic, SARS-CoV-2-positive women who were not separated from their mothers.
If a SARS-COV-2 negative, non-exposed adult is not available to care for the baby full time upon discharge, infant separation from the mother while inpatient is discouraged for the following reasons (Korraa, 2020):
- Molecular testing often remains positive for several weeks, making timing of re-unification difficult to determine in the absence of symptoms
- If the plan is for the newborn to be discharged with the mother, exposure will exist regardless, and she will need instruction in how to provide newborn care while using protective measures (mask wearing/hand hygiene)
- There are a number of practical and logistical challenges in discharging a newborn to a mother who has never cared for her infant
- Breastfeeding, if the chosen method of feeding, will be exceedingly difficult to establish if a SARS-CoV-2 positive mother is separated from her newborn
The AAP/CDC provide guidelines on alternative methods of “separation” including distancing within one’s room, physical barriers such as drapes and isolettes, and mask wearing. Any infant requiring more than level 1 (routine newborn) care is admitted to a single patient room (preferably negative pressure if receiving aerosol generating procedures) on enhanced respiratory precautions until cleared through molecular testing. There is some conflict amongst US and international medical societies regarding the recommendations of location of mother and infant in the immediate post-delivery period (WHO FAQ, 2020, AAP, 2020, ACOG PA, 2020, CDC, 2020, CPS, 2020). However, if maternal status allows it, it is reasonable to practice what will be done at home to help prepare for the safest practices after discharge.
Can SARS-CoV-2-positive Mothers Breastfeed?
A large body of evidence has established the benefits of breastfeeding -- this is beyond the scope of this FLARE and will not be discussed here (Bartick et al., 2017; of Obstetricians et al., 2016; Section on Breastfeeding, 2012; Victora et al., 2016). There is no data to suggest the presence of SARS-CoV-2 infection should influence a mother’s decision about feeding strategy.
Currently, it is unknown if SARS-CoV-2 infection can be transmitted by breast milk. Several small series and individual reports have evaluated human breast milk for SARS-CoV-2 RNA. In a small case series of 13 pregnant Chinese women, one of three breastmilk samples was positive for SARS-CoV-2 on PCR testing (Wu et al., 2020). In a different series of 26 mothers, all breast milk samples tested negative by PCR (Elshafeey et al., 2020). A pre-print, non-peer reviewed article examines 16 additional patients reported in a number of different studies: all of the breast milk samples tested negative for SARS-CoV-2 (Lackey et al., 2020). Clearly more data and rigorous testing are needed. We note that for other coronaviruses such as SARS-CoV or MERS-CoV, there is no evidence to suggest transmission via breast milk, although the number of published reports are even fewer than in SARS-CoV-2. Furthermore, there is no data to support that feeding expressed milk is “safer” than direct feeding. Shared decision making between each woman with SARS-CoV-2 and her healthcare team should occur as soon as possible after admission to establish a feeding plan. In general, there is agreement that breast milk appears to be safe at this time.
Conclusions
Data surrounding the care of SARS-CoV-2 infected pregnant women and their newborns are sparse, in contrast to the numerous questions asked by both clinicians and patients. As such, care for these vulnerable patients is predominantly guided by society recommendations and expert opinion. Currently, there is no clear data to suggest that providers should modify their recommendations for timing or mode of delivery based on the presence of SARS-CoV-2 infection. We await thorough serological analysis of mothers and neonates using validated IgG and IgM antibody tests to clarify the possibility of true vertical transmission of SARS-CoV-2. Decisions regarding separation of the mother-baby dyad, feeding approaches and discharge planning for SARS-CoV-2 positive mothers and their infants must involve a personalized approach.
References:
- Auger, N., Le, T.U.N., Park, A.L., and Luo, Z.-C. (2011). Association between maternal comorbidity and preterm birth by severity and clinical subtype: retrospective cohort study. BMC Pregnancy Childbirth 11, 67.
- Bartick, M.C., Schwarz, E.B., Green, B.D., Jegier, B.J., Reinhold, A.G., Colaizy, T.T., Bogen, D.L., Schaefer, A.J., and Stuebe, A.M. (2017). Suboptimal breastfeeding in the United States: Maternal and pediatric health outcomes and costs. Matern. Child Nutr. 13.
- Baud, D., Greub, G., Favre, G., Gengler, C., Jaton, K., Dubruc, E., and Pomar, L. (2020). Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection. JAMA.
- Breslin, N., Baptiste, C., Gyamfi-Bannerman, C., Miller, R., Martinez, R., Bernstein, K., Ring, L., Landau, R., Purisch, S., Friedman, A.M., et al. (2020). COVID-19 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM 100118.
- Chen, H., Guo, J., Wang, C., Luo, F., Yu, X., Zhang, W., Li, J., Zhao, D., Xu, D., Gong, Q., et al. (2020a). Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet 395, 809–815.
- Chen, L., Li, Q., Zheng, D., Jiang, H., Wei, Y., Zou, L., Feng, L., Xiong, G., Sun, G., Wang, H., et al. (2020b). Clinical Characteristics of Pregnant Women with Covid-19 in Wuhan, China. N. Engl. J. Med.
- Di Mascio, D., Khalil, A., Saccone, G., Rizzo, G., Buca, D., Liberati, M., Vecchiet, J., Nappi, L., Scambia, G., Berghella, V., et al. (2020). Outcome of Coronavirus spectrum infections (SARS, MERS, COVID 1 -19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 100107.
- Elshafeey, F., Magdi, R., Hindi, N., Elshebiny, M., Farrag, N., Mahdy, S., Sabbour, M., Gebril, S., Nasser, M., Kamel, M., et al. (2020). A systematic scoping review of COVID-19 during pregnancy and childbirth. Int. J. Gynaecol. Obstet.
- Hantoushzadeh, S., Shamshirsaz, A.A., Aleyasin, A., Seferovic, M.D., Aski, S.K., Arian, S.E., Pooransari, P., Ghotbizadeh, F., Aalipour, S., Soleimani, Z., et al. (2020). Maternal Death Due to COVID-19 Disease. Am. J. Obstet. Gynecol.
- Korraa., A. (2020). Management of Neonates Born to Mothers with COVID-19. Annals of Neonatology Journal 0, 0–0.
- Lackey, K.A., Pace, R.M., Williams, J.E., Bode, L., Donovan, S.M., Järvinen, K.M., Seppo, A., Raiten, D.J., Meehan, C.L., McGuire, M.A., et al. (2020). SARS-CoV-2 and human milk: what is the evidence? medRxiv 2020.04.07.20056812.
- of Obstetricians, A.C., Gynecologists, and Others (2016). Optimizing support for breastfeeding as part of obstetric practice. Committee Opinion No. 658. Obstet. Gynecol. 127, e86–e92.
- Ouzounian, J.G., and Elkayam, U. (2012). Physiologic changes during normal pregnancy and delivery. Cardiol. Clin. 30, 317–329.
- Pierce-Williams, R.A.M., Burd, J., Felder, L., Khoury, R., Bernstein, P.S., Avila, K., Penfield, C.A., Roman, A.S., DeBolt, C.A., Stone, J.L., et al. (2020). Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol MFM 100134.
- Schnettler, W.T., Al Ahwel, Y., and Suhag, A. (2020). Severe ARDS in COVID-19-infected pregnancy: obstetric and intensive care considerations. American Journal of Obstetrics & Gynecology MFM 100120.
- Section on Breastfeeding (2012). Breastfeeding and the use of human milk. Pediatrics 129, e827–e841.
- Society of Maternal Fetal Medicine, “Management Considerations for Pregnant Patients With COVID-19”, April 30, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2336/SMFM_COVID_Management_of_COVID_pos_preg_patients_4-30-20_final.pdf
- Sutton, D., Fuchs, K., D’Alton, M., and Goffman, D. (2020). Universal Screening for SARS-CoV-2 in Women Admitted for Delivery. N. Engl. J. Med.
- Vallejo, V., and Ilagan, J.G. (2020). A Postpartum Death Due to Coronavirus Disease 2019 (COVID-19) in the United States. Obstet. Gynecol.
- Victora, C.G., Bahl, R., Barros, A.J.D., França, G.V.A., Horton, S., Krasevec, J., Murch, S., Sankar, M.J., Walker, N., Rollins, N.C., et al. (2016). Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387, 475–490.
- Wu, Y., Liu, C., Dong, L., Zhang, C., Chen, Y., Liu, J., Zhang, C., Duan, C., Zhang, H., Mol, B.W., et al. (2020). Coronavirus disease 2019 among pregnant Chinese women: Case series data on the safety of vaginal birth and breastfeeding. BJOG.
- (2019). ACOG Practice Bulletin No. 211: Critical Care in Pregnancy. Obstet. Gynecol. 133, e303–e319.
View all COVID-19 Updates
Learn more about research in the Division of Pulmonary and Critical Care Medicine