The Long-awaited Remdesivir Trial
The FLARE Four
- Remdesivir is a nucleoside analog that inhibits the RNA-dependent RNA polymerase of SARS-CoV-2. Previous studies of remdesivir in COVID-19 have suffered from serious methodological flaws. ACTT-1 is a randomized, placebo-controlled trial of remdesivir in COVID-19
- In a preliminary analysis of ACTT-1, participants who received remdesivir had a shorter time to recovery than those who received placebo (11 days vs. 15 days)
- This benefit of remdesivir was observed among the subgroup of patients with serious, though not critical disease (those requiring oxygen, but not HFNC, NIPPV or mechanical ventilation)
- ACTT-1 did not enroll equally across all classes of disease severity, with fewer patients enrolled in low (no oxygen) and high (HFNC, NIPPV, mechanical ventilation) severity categories. Therefore, the trial may not have been adequately powered to detect benefit in other subgroups
Many people are asking...who would benefit from remdesivir?
Subscribe to the latest updates from FLARE Advances in Motion
Introduction
With millions of cases of SARS-CoV-2 infection and nearly 350,000 deaths worldwide due to COVID-19 to date, the need for specific antiviral therapies is imperative. Multiple repurposed or newly developed antiviral treatments have been proposed. Many treatments for COVID-19 have been described in case series and retrospective cohort studies (May 8 FLARE), but few have been tested in a randomized controlled trial (May 11 FLARE). In tonight's FLARE, we review the publication of the Adaptive COVID-19 Treatment Trial-1 (ACTT-1), an RCT that resulted in the emergency use authorization by the U.S. Food and Drug Administration (FDA) for remdesivir (Beigel et al. 2020).
Antiviral Therapy and Parallels to HIV
Antiviral therapies have been a part of our arsenal since idoxuridine was approved against herpesviruses in 1963. They are now used for preventive and therapeutic indications against a wide variety of DNA and RNA viruses (Figure 1). Previously, the time from discovery of the virus to the first effective therapy could be measured in years; technological advances and acute need have accelerated this timeline to just a few months for COVID-19.
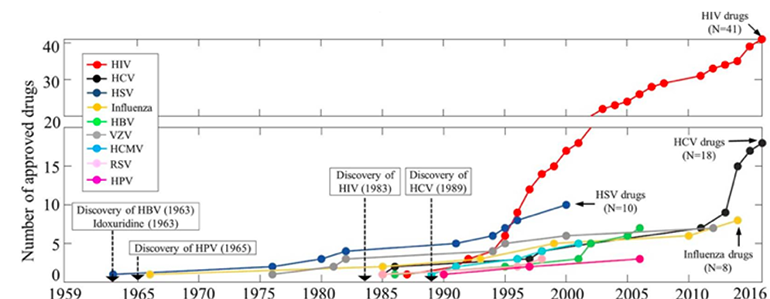
Figure 1
Timeline of approval of drugs against nine human infectious diseases (HIV, HBV, HCV, HSV, HCMV, HPV, RSV, VZV, and influenza virus). The x-axis indicates the period from January 1959 to April 2016, and the y-axis shows the total number of approved drugs (De Clercq and Li 2016).
Many who witnessed the early days of the HIV epidemic recall similarities to the early experience with COVID-19: the isolation of those suffering, the mounting caseload and death toll, the multitude of suggested but unproven treatments, and the feeling of helplessness amongst clinicians. Another evocative parallel is that the first proven treatment against HIV-1 was zidovudine (commonly known as AZT), a repurposed nucleoside analog, similar to a number of agents being studied for use against SARS-CoV-2. Dr. Anthony Fauci, the Director of the National Institute of Allergy and Infectious Diseases specifically referenced parallels between zidovudine to remdesivir during his announcement from the oval office on April 29th.
Let's Turn to Remdesivir
Remdesivir inhibits the RNA-dependent RNA polymerase of both Ebola and SARS-CoV-2. In the April 16th FLARE, we reviewed remdesivir’s unique mechanism of incorporation that bypasses the viral proofreading function, clinical data from its use for Ebola, and the first case series of compassionate use in 61 COVID-19 patients (Grein et al. 2020). Because the earlier study did not have a comparison group (and had statistical analysis flaws, which are now corrected in response to multiple letters to the editor) it was not possible to conclude anything about the effectiveness of remdesivir from the earlier publication (Grein, Myers, and Brainard 2020).
Insights From Rhesus Macaques
Rhesus macaques are considered a reliable animal model for COVID-19 (Munster et al. 2020) since they develop viral shedding and lower respiratory infection analogous to that seen in moderate COVID-19 in humans (de Wit et al. 2020). An unpublished study described two groups of six rhesus macaques inoculated with SARS-CoV-2 by multiple routes (Williamson et al. 2020). Starting 12 hours after inoculation, one group was given IV remdesivir and the other was treated with a control solution. Remdesivir-treated macaques demonstrated less radiographic burden of disease, tachypnea, and dyspnea. Interestingly there were no significant differences in viral loads or ability to isolate infectious viral particles from other sites including the nose, throat, or rectum, with the exception of infectious titers from bronchoalveolar lavage specimens. All animals were euthanized on day 7 and the treated animals had less pathologic and histologic evidence of disease with viral RNA found in fewer lung lobes compared with the control animals; infectious virus was not isolated from the remdesivir treated animals, in contrast to recovery of infectious virus in 5 of 6 control animals.
But Wait, Wasn't There an RCT for Remdesivir That Was Negative?
The first randomized data for remdesivir in humans was published in The Lancet on April 29, 2020 (Wang et al. 2020). Patients from 10 hospitals in China were randomized (2:1) in blinded fashion to receive either remdesivir or placebo. Participants had COVID-19 with hypoxemia and were within 12 days of symptom onset at time of randomization. Other treatments were allowed: at baseline, around 20% of patients in each group were on lopinavir/ritonavir and interferon alpha and around 40% were on corticosteroids, and during the trial application of these adjunctive therapies remained equal between the groups. The average time from symptom onset to starting treatment was balanced: 11 days in the remdesivir group and 10 days in the placebo group.
The primary outcome of the trial was time to clinical improvement on an ordinal scale or time to live discharge from the hospital (whichever came first). Investigators had a pre-specified goal to enroll 453 people to achieve adequate power, but no patients were enrolled after March 12, 2020 because the outbreak was controlled in Wuhan. They fell significantly short of their recruitment goal and ended up with 158 in the remdesivir arm and 78 in the placebo arm.
Overall there was no difference in time to clinical improvement or day 28-mortality. A subgroup analysis revealed a trend towards improved mortality among those started on remdesivir before day 10 of symptoms compared to those started later, but this was not statistically significant.
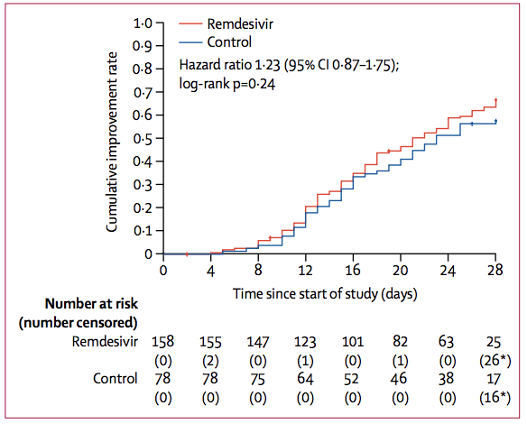
Figure 2
Time to clinical improvement in the intention-to-treat population (Wang et al. 2020).
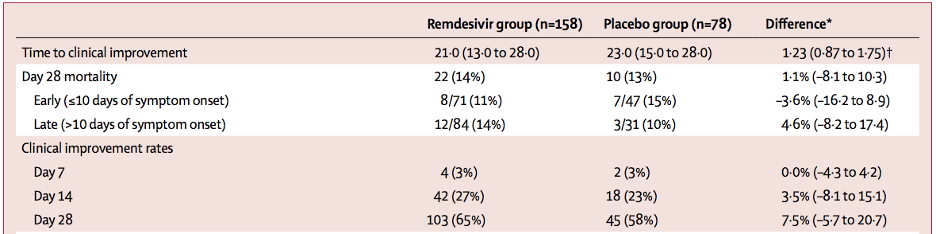
Table 1
Outcomes by the Intention-to-Treat Population (Wang et al. 2020).
They also found no difference in viral loads from the upper and lower respiratory tracts between the two groups. Importantly, isolation of infectious viral particles was not attempted. Ultimately, this study has numerous limitations, most importantly that it was underpowered. The extensive use of steroids and other antivirals are also confounding factors.
The Writing of ACTT-1
Just as the outbreak was receding in Wuhan, the pandemic accelerated in multiple other countries. In response, a total of 68 sites from North America, Europe, and Asia enrolled 1063 patients from February to April 2020 in ACTT-1 (Beigel et al. 2020). Participants were randomized 1:1 to receive up to 10 days of remdesivir versus placebo. The trial enrolled adults with COVID-19 who had one or more of the following characteristics: pulmonary infiltrates on imaging, oxygen saturation ≤ 94% on room air, need for supplemental oxygen, or mechanical ventilation. Patients with ALT or AST > 5 times the upper limit of normal or estimated glomerular filtration rate (eGFR) < 30 ml/min (initially < 50 ml/min) were excluded, as were women who were pregnant or breastfeeding.
How Do These Trials Classify Clinical Presentations and Outcomes?
COVID-19 may be asymptomatic, mild, moderate, severe (definitions vary, particularly by hypoxemia with differing cutoffs usually around ≤ 93-94% on room air, or requirement of supplemental oxygen), or critical (requiring mechanical ventilation and/or ECMO). An ordinal scale is often applied to track outcomes (see table).
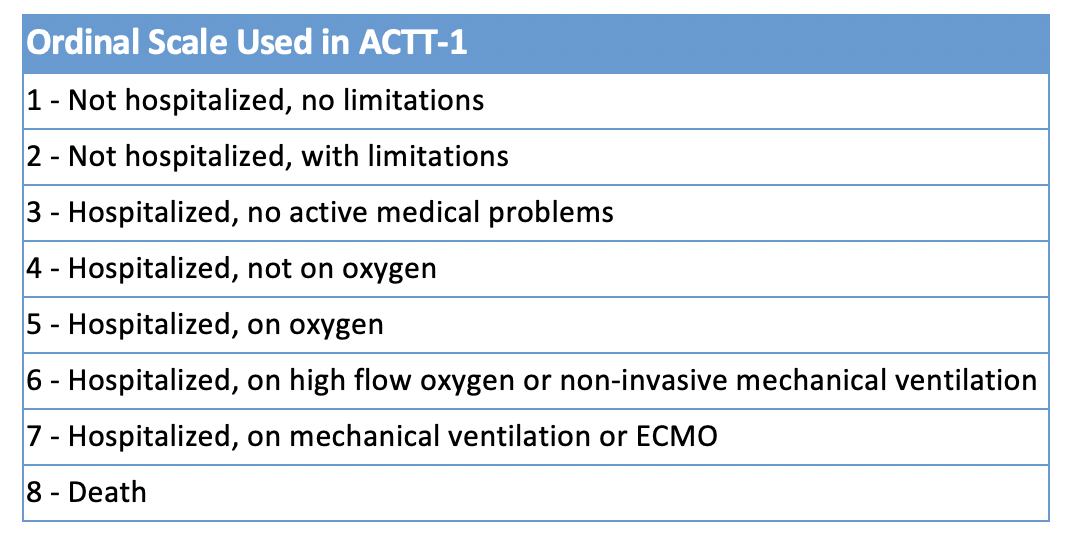
Table 2
Ordinal scale used in the international multisite study ACTT-1 (Beigel et al. 2020).
Initially, the primary endpoint was improvement on the ordinal scale at day 15, but once it was appreciated that many people with COVID-19 take longer to recover, the primary endpoint was changed to “time to recovery,” defined as the first day of reaching a score of 1, 2, or 3 on the ordinal scale. This change was proposed on March 22nd and the decision made on April 2nd, before any data were analyzed.
The most meaningful clinical outcome of any intervention would be mortality, but this was not the primary endpoint of ACTT-1. Mortality is a potentially inappropriate endpoint for this trial due to the relatively short follow-up. As reviewed in a prior FLARE (April 23), mortality numbers can be highly inaccurate when large numbers of patients have not yet reached a definitive outcome. Other outcomes that are likely to be meaningful are those related to utilization of resources (given the possibility of overwhelming health care systems) such as avoidance of intubation, days receiving mechanical ventilation or ECMO. The time to recovery to a state with low mortality risk is also a reasonable outcome.
Emergency Use Authorization and ACTT-1
Under normal circumstances, we would learn about a drug’s efficacy through a sequential evaluation of in vitro data, safety and early efficacy in animal and first in human studies, then an adequately powered RCT that precedes broader use after FDA approval. The urgency of the COVID-19 pandemic changed the above sequence, and until the publication of ACTT-1, we were practicing under the unusual circumstance of deploying remdesivir under an emergency use authorization (EUA) with only minimal preliminary results published.
On April 19, trial enrollment completed and on April 29 a press release was issued after an interim review by a Data Safety Monitoring Board. At the time of the interim review, < 50% of participants had completed 28 days of follow-up, so the analysis was based on a subset of participants. Participants who received remdesivir had more rapid time to recovery than those who received placebo: median of 11 days vs. 15 days. This precipitated the emergency use authorization on May 1, with language as follows: “...this authorization will be used only to treat adults and children with suspected or laboratory confirmed COVID-19 and severe disease defined as SpO2 ≤ 94% on room air, requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation.”
Three Weeks Later, We Learn More Details...
On May 22, 2020, the first publication from the ACTT trial was released (Beigel et al. 2020). The topline results were consistent with the prior statement (median 11 vs. 15 days, p < 0.001; rate ratio for recovery 1.32 [95% CI 1.12-1.55]). Mortality was 7.1% with remdesivir and 11.9% with placebo (hazard ratio for death, 0.70; 95% CI, 0.47 to 1.04). Even these data are preliminary as a significant number of patients (132 in the remdesivir group and 169 in the placebo group, or about 30% of the total population) have not yet recovered or completed the day 29 follow-up visit.
ACCT-1 recruited COVID-19 patients with radiographic infiltrates or those who required some amount of supplemental oxygen (defined as SpO2 ≤ 94% on room air, or requiring supplemental oxygen, mechanical ventilation, or ECMO). Notably, they enrolled over twice as many who required supplemental oxygen but not higher levels of support (Group 5, n = 421) in comparison to the trial from China (n = 194), allowing a greater power to test the hypothesis that remdesivir is beneficial in this subgroup.
What Was the Result?
As mentioned above, patients in the remdesivir arm had a shorter time to recovery than those in the placebo arm. As indicated in Figure 3 (adapted from Figure 2 of manuscript), patients on remdesivir reached 1, 2 or 3 on the ordinal scale (“recovered”) in a median time of 11 days, compared to a median time of 15 days in the placebo group. The authors also performed a subgroup analysis looking at the performance of remdesivir according to severity of illness. There was significant benefit in terms of earlier recovery in the group requiring oxygen, which was not apparent in patients receiving HFNC or NIPPV, mechanical ventilation or ECMO.
Does This Mean Patients Outside of Those Receiving Supplemental Oxygen Won’t Benefit From Remdesivir?
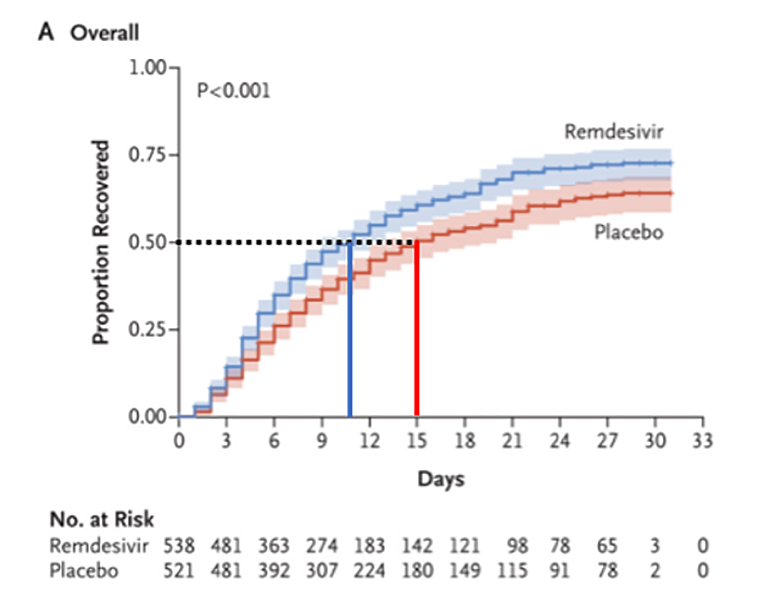
Figure 3
Kaplan-Meier curve of the proportion of patients recovered (Beigel et al. 2020).
Who Were the Patients?
Interpretation of this analysis is complicated by low numbers of patients in some subgroups. For example, there were 421 patients who required supplemental oxygen, 197 who required high flow nasal cannula or non-invasive positive pressure ventilation, and 272 who required mechanical ventilation or ECMO. This would suggest that the overall benefit is driven by the benefit in that subgroup requiring only supplemental oxygen. It remains possible that other groups benefited as well, though the trial was insufficiently powered to detect it.
Mortality—a Trend Is a Trend by Any Other Name
There is no significant mortality benefit in these data. However, it is worth noting that the trial was stopped early, with many outcomes not yet determined. It is possible that a better powered trial would show different results.
Safety
Serious adverse events (SAE) were reported for 114 of the 541 patients in the remdesivir group who underwent randomization (21.1%) and 141 of the 522 patients in the placebo group who underwent randomization (27.0%). No SAE occurred at a significantly different rate between groups. Unfortunately, no information was given regarding two subpopulations - how often remdesivir was stopped or held for elevated liver enzymes, or when patients experienced renal injury. Nevertheless safety does not appear to be a major limiting factor, outside of the cautions expressed in the EUA (excluding people with ALT ≥ 5x ULN, or eGFR < 30).
Limitations
The study was underpowered to show meaningful differences in certain subgroups, such those with mild-moderate disease or for those on ECMO. The authors did not report exposure to other therapies, such as corticosteroids (to facilitate comparison with the paper from China) or hydroxychloroquine. The report includes no data regarding viral markers, which would be useful to compare with the RCT from China and the rhesus macaque study. Finally, as patients with eGFR < 30 were excluded from ACTT-1, the benefit for this subpopulation must be weighed against the risk of toxicity of the cyclodextrin vehicle, especially in those with acute kidney injury but without renal replacement therapy.
5 or 10 days?
Results of a separate manufacturer-sponsored trial (called SIMPLE) have been reported in a press release. This trial enrolled 397 participants with severe COVID-19 but excluded those who were mechanically ventilated. Participants were randomized 1:1 to receive 5 or 10 days of remdesivir. The overall conclusion of this trial is that outcomes after 5 days of remdesivir were not statistically significantly different than those after 10 days of remdesivir for people with severe COVID-19. As this study did not enroll people who were mechanically ventilated, the FDA EUA for remdesivir suggests 10 days of therapy for this population. We await full release of these data.
So...Who Would Benefit Most?
We don’t know with certainty. The subgroup in ACTT-1 in which both the sample size and effect size converge to show a meaningful impact (Iwashyna et al. 2015) of remdesivir is composed of those on supplemental oxygen who are not intubated. Given power limitations, however, ACCT-1 neither demonstrated nor disproved a potential benefit of remdesivir for the categories of patients less sick or more sick that those on supplementary oxygen. The safest conclusion at this point is that the preliminary data do not show a benefit of remdesivir in people who are mechanically ventilated but this group may be underpowered.
Conclusions
To quote a colleague, remdesivir’s reduction of time to recovery for COVID-19 patients is not a “home run,” but a “solid stand-up double.” In particular, the trial demonstrates maximal benefit of remdesivir for hospitalized patients who are on supplemental oxygen but did not prove benefit for those on HFNC, NIPPV, invasive mechanical ventilation, or ECMO. In the setting of limited supplies of remdesivir, patients most likely to derive benefit should be the highest priority for allocation and these are the best data to guide that decision currently.
The next question is how to string together more hits to rally against this virus. Combination with other antivirals may be considered, as this approach was effective against HIV and HCV. The next “act” for the NIH group will be testing remdesivir with or without baracitinib, a Janus Kinase inhibitor with postulated antiviral effects and immunomodulatory properties (Stebbing et al. 2020).
It took years between the discovery of HIV to find a medication (AZT) that had an effect on outcomes and many more years to develop combinations of medications that meaningfully extended the lifespan of people with HIV. The finding that remdesivir improves time to recovery gives us a foundation to build on -- with the hope that progress will be measured in months, not years, to turn the tide against COVID-19.
References
- Beigel, John H., Kay M. Tomashek, Lori E. Dodd, Aneesh K. Mehta, Barry S. Zingman, Andre C. Kalil, Elizabeth Hohmann, et al. 2020. “Remdesivir for the Treatment of Covid-19 - Preliminary Report.” The New England Journal of Medicine, May. https://doi.org/10.1056/NEJMoa2007764.
- De Clercq, Erik, and Guangdi Li. 2016. “Approved Antiviral Drugs over the Past 50 Years.” Clinical Microbiology Reviews 29 (3): 695–747.
- Grein, Jonathan, Robert P. Myers, and Diana Brainard. 2020. “Compassionate Use of Remdesivir in Covid-19. Reply.” The New England Journal of Medicine. https://doi.org/10.1056/NEJMc2015312.
- Grein, Jonathan, Norio Ohmagari, Daniel Shin, George Diaz, Erika Asperges, Antonella Castagna, Torsten Feldt, et al. 2020. “Compassionate Use of Remdesivir for Patients with Severe Covid-19.” The New England Journal of Medicine, April. https://doi.org/10.1056/NEJMoa2007016.
- Iwashyna, Theodore J., James F. Burke, Jeremy B. Sussman, Hallie C. Prescott, Rodney A. Hayward, and Derek C. Angus. 2015. “Implications of Heterogeneity of Treatment Effect for Reporting and Analysis of Randomized Trials in Critical Care.” American Journal of Respiratory and Critical Care Medicine 192 (9): 1045–51.
- Munster, Vincent J., Friederike Feldmann, Brandi N. Williamson, Neeltje van Doremalen, Lizzette Pérez-Pérez, Jonathan Schulz, Kimberly Meade-White, et al. 2020. “Respiratory Disease in Rhesus Macaques Inoculated with SARS-CoV-2.” Nature, May. https://doi.org/10.1038/s41586-020-2324-7.
- Stebbing, Justin, Anne Phelan, Ivan Griffin, Catherine Tucker, Olly Oechsle, Dan Smith, and Peter Richardson. 2020. “COVID-19: Combining Antiviral and Anti-Inflammatory Treatments.” The Lancet Infectious Diseases.
- Wang, Yeming, Dingyu Zhang, Guanhua Du, Ronghui Du, Jianping Zhao, Yang Jin, Shouzhi Fu, et al. 2020. “Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial.” The Lancet 395 (10236): 1569–78.
- Williamson, Brandi N., Friederike Feldmann, Benjamin Schwarz, Kimberly Meade-White, Danielle P. Porter, Jonathan Schulz, Neeltje van Doremalen, et al. 2020. “Clinical Benefit of Remdesivir in Rhesus Macaques Infected with SARS-CoV-2.” bioRxiv. https://doi.org/10.1101/2020.04.15.043166.
- Wit, Emmie de, Friederike Feldmann, Jacqueline Cronin, Robert Jordan, Atsushi Okumura, Tina Thomas, Dana Scott, Tomas Cihlar, and Heinz Feldmann. 2020. “Prophylactic and Therapeutic Remdesivir (GS-5734) Treatment in the Rhesus Macaque Model of MERS-CoV Infection.” Proceedings of the National Academy of Sciences of the United States of America 117 (12): 6771–76.
Learn more about research in the Division of Pulmonary and Critical Care Medicine
View all COVID-19 Updates