Connectome 2.0: Revealing Structural Connections in the Brain with the Most Advanced Scanner for Diffusion MRI
In This Article
- The Martinos Center for Biomedical Imaging at Massachusetts General Hospital has installed "Connectome 2.0," a state-of-the-art MRI scanner for imaging structural connections within the human brain
- The scanner will play an integral role in the work of the new Center for Large-scale Imaging of Neural Circuits (LINC), a five-year, $23.5 million project supported by the NIH BRAIN Initiative and based in part at the Martinos Center
- With the launch of the scanner, researchers have also introduced an effort to increase diversity among subjects in studies with the scanner and ensure that the benefits of the technology are shared equitably across the communities it is meant to serve
In July 2023, the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital saw the installation of the latest state-of-the-art, high-performance MRI scanner for imaging the human brain's wiring. Dubbed "Connectome 2.0," the system is set to provide unprecedented insights into the inner workings of the living human brain, with important implications for improving the diagnosis and treatment of neurological and mental disorders.
Subscribe to the latest updates from Radiology Advances in Motion
Imaging of the human "connectome," as the complete set of connections within the brain has come to be known, dates to the mid-1990s when Martinos Center researchers pioneered the MR imaging methods that made such imaging possible. "Diffusion" imaging enables imaging of axonal bundles—the wires that facilitate communication between different brain areas—by tracking water molecules as they travel along these white matter fibers. By the mid-2000s, the researchers had advanced the technology to the point where they could discern tightly packed fibers running in different directions and often crossing over one another.
Based on this trailblazing work, in September 2010, as part of the $40 million Human Connectome Project, the National Institutes of Health chose the Martinos Center to help build a next-generation MRI scanner to enable diffusion imaging in humans. Here, in collaboration with the Laboratory of Neuro Imaging at the University of California, Los Angeles (now at the University of Southern California), and engineers at Siemens Healthcare, Martinos Center researchers developed a 3T MRI scanner that would offer the sensitivity and resolution needed to map white matter connectivity in the human brain.
Installation of the original "Connectome" MRI scanner at the Martinos Center was completed in September 2011, and researchers wasted no time in taking advantage of its many capabilities. Van Wedeen, PhD, one of the Martinos investigators who pioneered the diffusion spectrum imaging technique, reported in the journal Science a groundbreaking study showing a remarkable grid-like structure of fiber pathways in the brain, offering a new and utterly intriguing portrait of how the brain is organized. Study after study followed, revealing new relationships between different brain regions and advancing basic science research and other applications with clinical relevance.
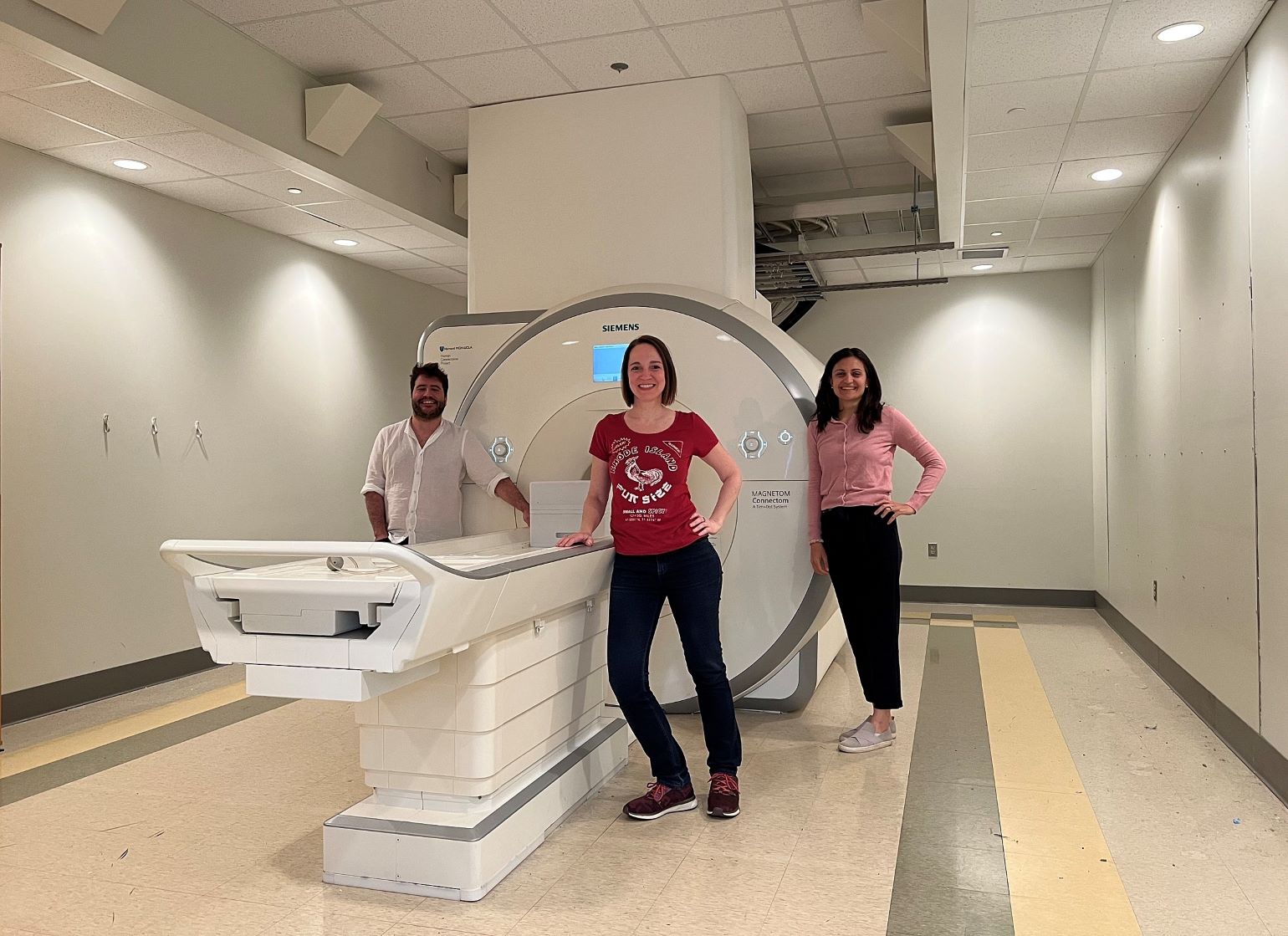
Figure 1
Anastasia Yendiki (center) with Gabriel Ramos Llorden (left), Chiara Maffei (right) and Hansol Lee (not shown) completed the last-ever scan on the Connectome 1.0 scanner on Thursday, May 25. Image courtesy of Anastasia Yendiki (Martinos Center).
But even as the scanner offered profound insights into the structural connections of the living human brain at the macroscopic level, it also shed light on the potential of a dedicated high-performance scanner to explore in vivo the microstructure of neural tissue—offering the tantalizing possibility of providing histological level information in the living human brain. Recognizing this potential, the Martinos-based team developed a next-generation Connectome MRI scanner for diffusion imaging of the brain: the now-installed Connectome 2.0.
"It's an amazing device," says Bruce Rosen, MD, PhD, director of the Martinos Center for Biomedical Imaging and vice-chair for research in the Mass General Department of Radiology. "It's more than four times as powerful as the previous Connectome scanner, which was almost five times as powerful as anything else on the planet before we developed it."
Susie Huang, MD, PhD, a neuroradiologist at Mass General and the Martinos faculty member leading the Connectome 2.0 effort, is thrilled about the potential applications of the technology.
"Connectome 2.0 will dramatically enhance our ability to visualize the structure of the human brain by offering unprecedented spatial and diffusion resolution," she says. "We are already seeing how the increased sensitivity of Connectome 2.0 to axonal and cellular structure at the micron level is allowing us to see surprising differences in how the human brain is organized at the individual level."
Dr. Huang and the team of investigators she has led believe that, as a research tool capable of mapping the microscopic, mesoscopic, and macroscopic connections in the brain, this scanner will help transform the scientific understanding of human neural circuitry and enable exploration of the microstructural substrate of functional neural plasticity.
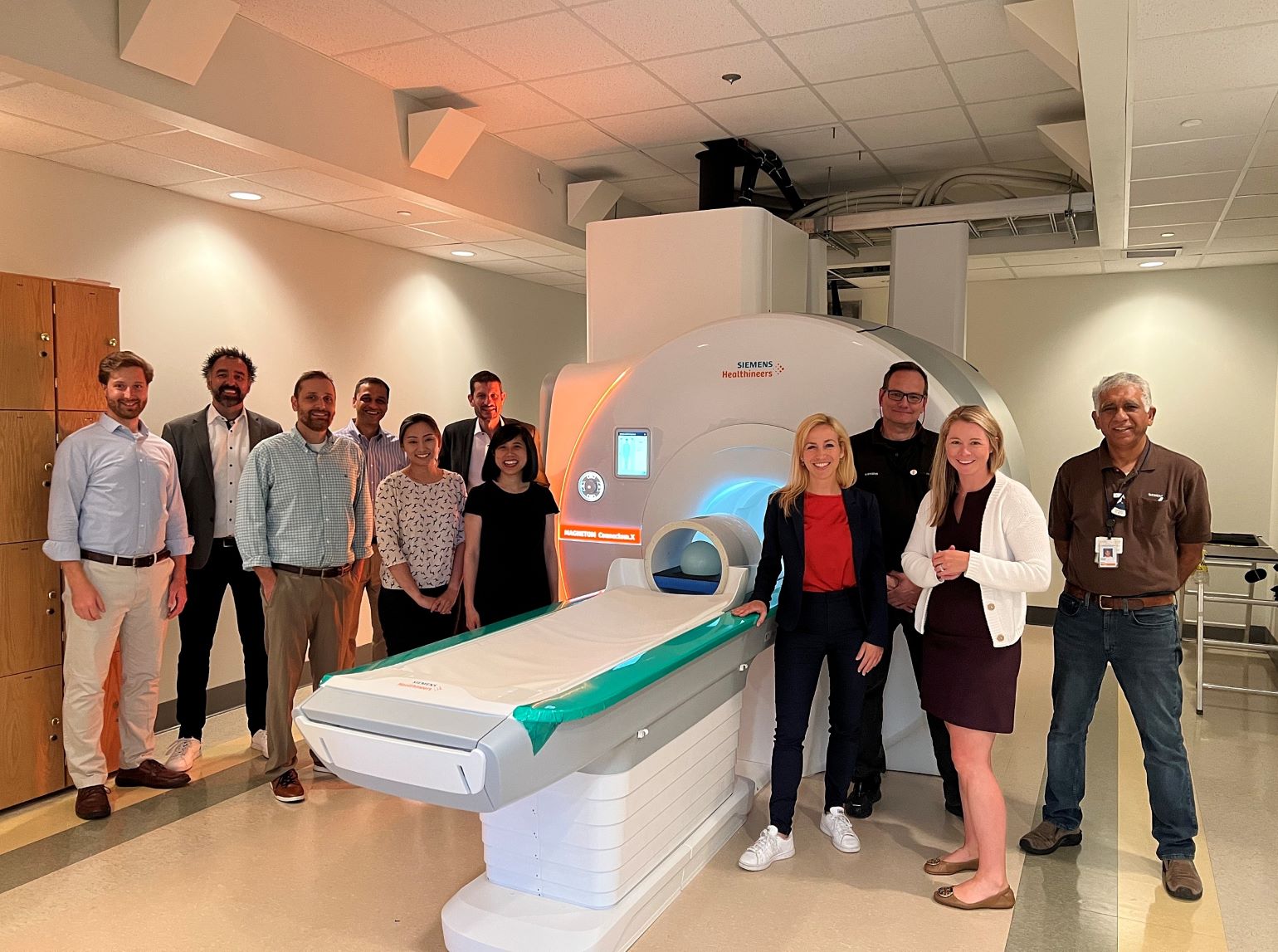
Figure 2
Siemens/Mass General team members with Connectome 2.0 on July 20, 2023. Image courtesy of Susie Huang (Martinos Center).
Diffusion Imaging Could Benefit Diagnosis and Treatment of Mental Disorders
Martinos Center researcher Anastasia Yendiki, PhD, has been a key user of the Center's two Connectome scanners from the start. In 2015, she and colleagues launched the Boston Adolescent Neuroimaging of Depression and Anxiety (BANDA) study to map the neural pathways in a population of adolescents with anxiety disorders and depression.
"Our understanding of the biological mechanisms of mental illness is still limited," she said several years later when the study was still ongoing. "This makes it very challenging to predict which treatment will work for which patient. We hope that, by mapping the brain signatures of depression and anxiety disorders at an age that is critical for brain development, we can discover reliable biomarkers that will allow doctors to perform accurate diagnoses and prescribe appropriate treatments for patients."
Initial findings from the study showed that, while there were alterations in both the structure and function of the nucleus accumbens in study participants, only a decrease in the volume of the nucleus accumbens predicted the development of depressive symptoms over time. The research team is exploring these alterations further to understand better how they might contribute to diagnosis and management of depression and anxiety in adolescents.
With Connectome 2.0 now installed at the Center, Dr. Yendiki has undertaken another study using diffusion imaging to probe connections within the brain—again with the goal of advancing clinical care.
Dr. Yendiki is the lead principal investigator of the new Center for Large-scale Imaging of Neural Circuits (LINC), a five-year, $23.5 million project supported by the NIH by way of the BRAIN Initiative Connectivity Across Scales (CONNECTS) program. Her co-PIs are Suzanne Haber, PhD, at the University of Rochester, and Elizabeth Hillman, PhD, at Columbia University.
The goal of the center is to develop innovative technologies with which to image connections in the brain down to the microscopic scale and apply the technologies to improve the accuracy of deep brain stimulation (DBS) for motor disorders, such as Parkinson's disease, Tourette syndrome, and dystonia, as well as psychiatric disorders such as obsessive-compulsive disorder (OCD) and depression.
"We know that deep brain stimulation works, but we are not exactly clear how it works," Dr. Yendiki says. "We are going to map these connections in human brains at a microscopic scale for the first time to give clinicians a clear picture of how brain regions are interconnected—and hopefully improve how these diseases are targeted."
Another BRAIN CONNECTS grant at the Center will also take advantage of Connectome 2.0.
Bruce Fischl, PhD, is director of the Laboratory for Computational Neuroimaging (LCN), which has developed and continually refined image analysis tools over the past 20-plus years, including FreeSurfer, an open-source software suite for processing, analyzing, and visualizing structural, functional and diffusion neuroimaging data.
With the recently awarded BRAIN CONNECTS grant "Mapping Connectivity of the Human Brainstem in a Nuclear Coordinate System," Dr. Fischl and colleagues will leverage this experience to develop technologies to create a multiscale atlas for the human brainstem. Call it a Google Earth for the brainstem. Using the atlas, researchers will be able to visualize brainstem-wide networks, then zoom in to the level of individual cells, probing their connectivity at micrometer resolution.
The new tool will enable types of analyses of neuroimaging data that simply haven't been possible before—and thus could lead to important advances in understanding traumatic brain injury, Alzheimer's disease, and a range of other neurological conditions.
Increasing Diversity, and Improving Equity, in Human Neuroimaging Research
With the launch of the Connectome 2.0 effort, Martinos Center researchers have also set their sights on a longstanding challenge in neuroscience research: how to increase diversity in the recruitment and enrollment of human participants, a critical step in improving the robustness of neuroscience research findings, and in ensuring that the benefits of new medical technology are shared equitably across the communities the technology is meant to serve.

Figure 3
The Martinos-based Connectome 2.0 team: (from left to right) Hong-Hsi Lee, Yixin Ma, Gabriel Ramos-Llorden, Mirsad Mahmutovic, Boris Keil, Anastasia Yendiki, Susie Huang. Image courtesy of Susie Huang (Martinos Center).
To this end, Dr. Huang is partnering with colleagues at the Harvard Medical School Center for Bioethics, Mass General Center for Law, Brain & Behavior (CLBB), and the Mass General Community Access, Recruitment, and Engagement (CARE) Center in a new project: Recruitment, Engagement, and Access for Community Health Equity for BRAIN Next-Generation Human Neuroimaging Research and Beyond (REACH for BRAIN).
Recently funded by the NIH BRAIN Neuroethics program, REACH for BRAIN will strive to improve engagement with and recruitment of research participants in studies using the Connectome 2.0 scanner.
"Neuroimaging technologies have improved dramatically over the years, but engagement and recruitment efforts have not," says Francis X. Shen, JD, PhD, a faculty member in the Harvard Medical School Center for Bioethics, and chief innovation officer at CLBB, and one of the co-PIs of the REACH for BRAIN project. "The main thrust of the project is to challenge and reimagine the ways neuroimaging research is conducted by grounding it in the local community,"
The project will introduce two major innovations. The first will be the creation of an engagement and recruitment infrastructure employing a "Theory of Change" framework pioneered by Helen Hemley, MPA, administrative director of the CARE Research Center. Using this framework will enable the team to develop "community-entrenched pathways" for individuals from underrepresented minority (URM) populations to engage and participate in Connectome 2.0 studies, ultimately allowing diverse non-researchers to contribute to procedures and technologies devised in the wake of the studies, beyond merely serving as research participants.
The second major innovation in the REACH for BRAIN project will be the development of neuroethical guidance covering measurement, reporting, and sharing of neuroimaging data with race and ethnicity identifiers. To help develop this guidance, the project will establish a national expert working group with diverse expertise in neuroscience and ethics.
Jonathan Jackson, PhD, executive director of the CARE Center and a co-PI of REACH for BRAIN, describes the impetus for this part of the project. "When it comes to cutting-edge practices and emerging technologies in medical science, historically marginalized populations are often left out or, worse, exploited," he says. "With REACH for BRAIN, we will learn from centuries of mistakes in brain research and invite marginalized groups to co-design—and co-own—research processes from the very beginning. Our hope is to find ways to limit or prevent racialized or essentialized questions from coming to the fore."
The innovations introduced by REACH for BRAIN are long overdue in neuroscience and, moving forward, will play a critical role in neuroimaging studies, especially as those studies delve deeper into the brain while expanding into new areas of research.
Dr. Huang, the Martinos-based co-PI of the project and the leader of the Connectome 2.0 effort, elaborates.
"The initial data we have obtained on Connectome 2.0 show that we can now detect tissue microstructural features within a single individual that would require dozens of measurements on standard clinical MRI scanners," she says. "These results suggest that Connectome 2.0 and other technologies supported by the NIH BRAIN Initiative will pave the way toward precision human neuroscience and thus underscore the importance of having greater representation and inclusion of participants as we make new discoveries using these amazing new tools. Furthermore, we will amplify the reach of the technology and inclusive frameworks we are developing in REACH for BRAIN by setting up the Connectome 2.0 scanner as a shared resource facility for scientists worldwide to take their experiments to new heights."
"This is exactly what REACH for BRAIN hopes to accomplish. I am very excited about partnering with our community stakeholders to ensure that this next-generation technology benefits all participants and increases the richness and diversity of insights afforded by Connectome 2.0 and other next-generation technologies developed at the Martinos Center and elsewhere."
Learn more about the Martinos Center for Biomedical Imaging
Learn more about the Large-scale Imaging of Neural Circuits (LINC) Center