Use of Convalescent Plasma
The FLARE Four
- The rationale and evidence for utilizing convalescent plasma in COVID-19
- Convalescent blood products have long been used to treat infection and have been studied during several outbreaks of novel pathogens during the 21st century
- Current trials and biotech developments
- The logistical requirements for administering convalescent plasma include five factors
Today's FLARE will investigate the rationale and evidence for utilizing convalescent plasma in COVID-19.
Subscribe to the latest updates from FLARE Advances in Motion
Why Might Convalescent Plasma Be Used to Treat Infections?
Convalescent blood products are among the oldest tools for treating infection in humans, dating back at least to the 1880s and used widely until the antibiotic era (1,2).
In vivo data in HIV infection suggests that the passive immunity conferred via antibodies from convalescent plasma may both promote free viral clearance and facilitate killing of infected host cells by Fcγ receptor-mediated mechanisms (3). Viremia peaks in the first 7 days in most viral illnesses, while a robust humoral immune response requires 10-14 days (4). Therefore, administration of convalescent plasma early in the course of infection may enhance viremic control pending the host adaptive immune response. However, it remains less clear whether analogous strategies would be effective in viral illnesses tropic to the lung, such as SARS-CoV-2.
What Prior Data Exist for the Use of Convalescent Plasma in Respiratory Viral Infections?
While the evidence supporting convalescent plasma administration is limited, several reports present promising data in SARS-CoV, mixed results in H1N1 influenza, and negative results in Ebola. A 2014 meta-analysis of 32 studies of SARS-CoV and influenza infections revealed consistent evidence for reduced mortality, especially when convalescent plasma was administered soon after symptom onset. However, these studies were mostly of low quality, lacked control groups, and were at moderate to high risk of bias (5). A non-peer-reviewed meta-analysis of convalescent plasma administration in influenza did not show improved mortality or consistently decreased viral loads (6). A table of significant trials is below:
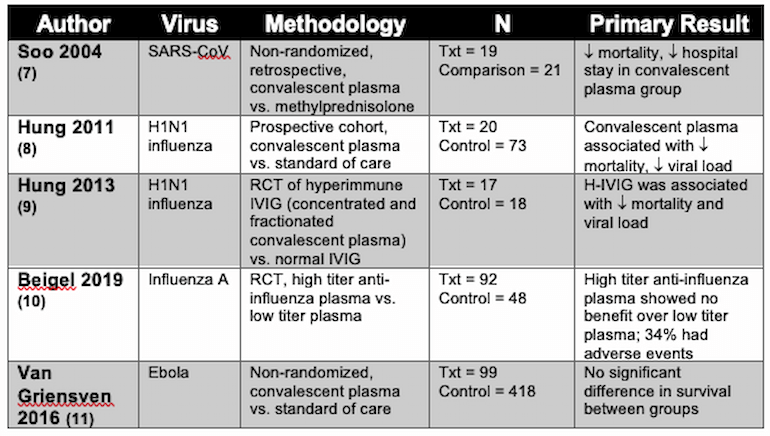
Table 1
What are the Data in SARS-CoV-2?
Convalescent plasma is currently recommended by the Chinese CDC for treatment of SARS-CoV-2 (12), and the United States FDA allows providers to seek emergency Investigational New Drug Applications for individual patients in life-threatening circumstances (13). A recent JAMA article (published the day of this writing) presents a case series of 5 critically-ill patients with PCR-confirmed SARS-CoV-2 infection, ARDS, and a high viral loads treated with 2 transfusions of ABO-compatible convalescent plasma as well as various combinations of antiviral agents (including lopinavir/ritonavir, IFN-alpha-1b, favipiravir, arbidol, darunavir). Donors were patients who (1) had recovered from SARS-CoV-2 infection, (2) tested negative for current SARS-CoV-2, other respiratory viruses, HIV, HBV and HCV, (3) were asymptomatic for at least ten days, and (4) had SARS-CoV-2 ELISA antibody antibody titer greater than 1:1000 and a neutralizing antibody titer greater than 1:40. Convalescent plasma was administered between days 10-22 of hospitalization. Viral load (estimated by PCR cycle threshold) improved by 1 day after transfusion, with all 5 patients becoming undetectable by PCR after 12 days. For all patients, SOFA score, P/F ratios, and temperatures improved. Three of the 5 patients were discharged home on hospital days 51-53, while 2 were improving, but still on mechanical ventilation at the time of publication. This non-randomized cohort suggests some potential promise and further study is needed to determine efficacy (14).
What are the Limitations of Using Convalescent Plasma for Treatment of SARS-CoV-2?
There are multiple logistical challenges which must be overcome to provide this therapy to COVID-19 patients on a large scale.
First, the procurement and provision of convalescent plasma is resource-intensive and expensive. Second, supply of convalescent serum may be problematic. Not all persons who recover from viral illness develop high serum titers of neutralizing antibodies during the convalescent phase; Zhang et al analyzed convalescent serum from 99 SARS-CoV patients and found only 87 had positive neutralizing antibody titers 35-180 days after symptom onset (15,16). Third, serologic tests to detect immunity from SARS-CoV-2 are only just being developed, which limits the ability of clinicians to identify those who could be candidates to donate. Fourth, it is hypothesized that convalescent plasma would be most beneficial to patients if given as soon as possible after symptom onset to stave off downstream severe illness, or even prophylactically in high-risk populations such as those with cardiovascular disease, immunosuppression, or healthcare workers (5,17). However, if the resource is not widely available, it would potentially be restricted to the sickest patients in critical care settings, who may benefit less. As such, clear guidelines and discussions about appropriate candidates will be needed. Lastly, convalescent plasma transfusion carries similar risks of transfusion of other blood products including infection, fever, transfusion reactions and TRALI (17,18).
What are the Logistical Requirements for Administering Convalescent Plasma? (17)
- Availability of donors who have recovered and are willing and able to donate plasma
- Blood bank infrastructure and transfusion specialists to prepare and administer plasma
- Availability of serological and viral assays and labs to measure for SARS CoV-2, to confirm viral clearance and detect antibody
- Therapeutic protocols for the use of the plasma
- Ethical considerations and regulatory oversight, including, but not limited, to IRB approval for clinical trials
Current Trials and Biotech Developments
Takeda Pharmaceuticals has already begun work on antibody preparations against SARS-CoV-2, but these preparations are unlikely to be available for many months (19). In addition to direct convalescent plasma from donors and concentrated antibody products such as Takeda’s, there is also potential for other means of producing antibodies for therapies via transgenic animal models. This approach was studied in MERS by Beigel et al. who reported a phase I trial of a human polyclonal IgG antibody produced from the plasma of transchromosomic cattle (transgenic animals with humanized chromosomes) (20).
As of the publishing of this document, there are 3 trials listed on clinicaltrials.gov investigating convalescent plasma in COVID-19 patients. One, in China, has started enrolling, while 2 others have not started recruiting subjects.
To Sum It All Up
Convalescent blood products have long been used to treat infection and have been studied during several outbreaks of novel pathogens during the 21st century including SARS-CoV, MERS, H1N1 influenza, and Ebola. The evidence is mixed and of generally poor quality, but most promising in SARS-CoV. A recent case series provides the first evidence of benefit in the current SARS-CoV-2 pandemic. There are many logistical challenges in developing and distributing convalescent sera products for treatment of SARS-CoV-2. We look forward to data from clinical trials to best determine the utility of this therapy.
References
- Marano G, Vaglio S, Pupella S, et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus 2016;14(2):152–7.
- McGuire LW, Redden WR. THE USE OF CONVALESCENT HUMAN SERUM IN INFLUENZA PNEUMONIA—A PRELIMINARY REPORT. Am J Public Health 1918;8(10):741–4.
- 3. Lu C-L, Murakowski DK, Bournazos S, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 2016;352(6288):1001–4.
- Cheng Y, Wong R, Soo YOY, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 2005;24(1):44–6.
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis 2015;211(1):80–90.
- Xu Z, Zhou J, Huang Y, et al. The efficacy of convalescent plasma for the treatment of severe influenza [Internet]. Infectious Diseases (except HIV/AIDS). 2020; Available from: http://dx.doi.org/10.1101/2020.02.20.20025593
- Soo YOY, Cheng Y, Wong R, et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect 2004;10(7):676–8.
- Hung IF, To KK, Lee C-K, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis 2011;52(4):447–56.
- Hung IFN, To KKW, Lee C-K, et al. Hyperimmune IV Immunoglobulin Treatment [Internet]. Chest. 2013;144(2):464–73. Available from: http://dx.doi.org/10.1378/chest.12-2907
- Beigel JH, Aga E, Elie-Turenne M-C, et al. Anti-influenza immune plasma for the treatment of patients with severe influenza A: a randomised, double-blind, phase 3 trial. Lancet Respir Med 2019;7(11):941–50.
- van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. N Engl J Med 2016;374(1):33–42.
- Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis [Internet] 2020; Available from: http://dx.doi.org/10.1016/S1473-3099(20)30141-9
- Center for Biologics Evaluation, Research. Investigational COVID-19 Convalescent Plasma - Emergency INDs [Internet]. U.S. Food and Drug Administration. 2020 [cited 2020 Mar 27]; Available from: http://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma-emergency-inds
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma [Internet]. JAMA. 2020;Available from: http://dx.doi.org/10.1001/jama.2020.4783
- Arabi YM, Hajeer AH, Luke T, et al. Feasibility of Using Convalescent Plasma Immunotherapy for MERS-CoV Infection, Saudi Arabia. Emerg Infect Dis 2016;22(9):1554–61.
- Zhang J-S, Chen J-T, Liu Y-X, et al. A serological survey on neutralizing antibody titer of SARS convalescent sera. J Med Virol 2005;77(2):147–50.
- Casadevall A, Pirofski L-A. The convalescent sera option for containing COVID-19. J Clin Invest [Internet] 2020; Available from: http://dx.doi.org/10.1172/JCI138003
- Gajic O, Rana R, Winters JL, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med 2007;176(9):886–91.
- Hopkins JS. Drugmaker Takeda Is Working on Coronavirus Drug [Internet]. WSJ Online. 2020 [cited 2020 Mar 27]; Available from: https://www.wsj.com/articles/drugmaker-takeda-is-working-on-coronavirus-drug-11583301660
- Beigel JH, Voell J, Kumar P, et al. Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study. Lancet Infect Dis 2018;18(4):410–8.
View all COVID-19 updates
Learn about research in the Division of Pulmonary and Critical Care Medicine