Of ACEs, ARBs and COVID-19
The FLARE Four
- Concern has been raised about the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin-receptor blockers (ARBs) and angiotensin receptor-neprilysin inhibitors (ARNis) during COVID-19 infection
- In this FLARE, we will review the basic biology of the ACE2 pathway, explore the existing data regarding ACE2 and lung injury, and consider implications for patients receiving these medications in the setting of COVID-19
- Classically thought of as a pathway to modulate salt-water balance and sympathetic tone, the RAAS pathway also includes a mechanism for vasodilation
- There is much to learn about ACE2, ACE/ACE2 balance in lung injury, SARS-CoV and SARS-CoV-2 coronavirus
Prior to tonight's installment, we would like to first acknowledge the authorship of yesterday's piece on the use of convalescent plasma. This was contributed by Dr. Laura Brenner and Dr. Tiara Calhoun.
Subscribe to the latest updates from FLARE Advances in Motion
Concern has been raised about the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin-receptor blockers (ARBs) and angiotensin receptor-neprilysin inhibitors (ARNis) during COVID-19 infection.
In this FLARE, we will review the basic biology of the ACE2 pathway, explore the existing data regarding ACE2 and lung injury, and consider implications for patients receiving these medications in the setting of COVID-19.
Background: Why Consider ACEs, ARBs and ANRIs During COVID-19 Infection?
Early reports about patients infected with SARS-CoV-2 revealed that the most common comorbidities of patients hospitalized with COVID-19 were cardiovascular diseases including hypertension, cerebrovascular disease, coronary artery disease or risk factors for cardiovascular disease such as diabetes. A selection of these reports is summarized below:
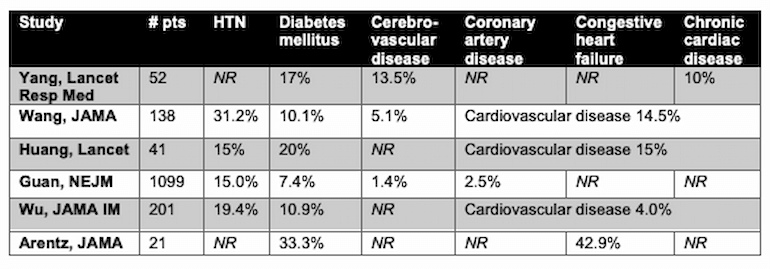
Table 1
Table modified from Li 2020. HTN = hypertension, NR = not reported.
Others have shown that patients with these comorbidities have higher rates of severe COVID-19, including ARDS and death (Wu 2020, Zhou 2020, Shi 2020). Additionally, a recent meta-analysis (Li 2020) including 1527 patients demonstrated that SARS-CoV-2 patients with these cardiovascular comorbidities experienced significantly higher rates of severe disease and/or ICU admission:
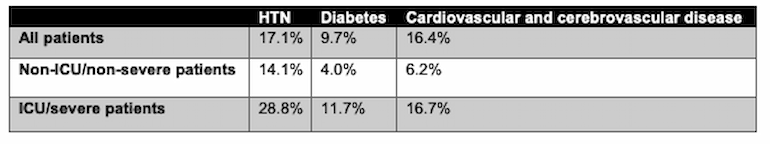
Table 2
Modified from Li 2020. HTN = hypertension.
These observations, along with a ongoing research on the role of ACE2 in SARS-CoV-2 infection, led to a provocative question posed by Feng et al. in Lancet Respiratory Medicine: could increased expression of ACE2 from chronic ACEi/ARB therapy facilitate SARS-CoV-2 infection (Fang 2020)? We explore this question below.
Basic Biology of ACE2 Pathway
Classically thought of as a pathway to modulate salt-water balance and sympathetic tone, the RAAS pathway also includes a mechanism for vasodilation. This is achieved by negative regulation via ACE2, an integral membrane enzyme. As shown in the figure below, ACE2 degrades angiotensin II into angiotensin 1-7 (Ang 1-7) and angiotensin 1-9 (Zhang 2017). Ang 1-7 activates the Mas receptor, which leads to vasodilatory, antiproliferative, and antifibrotic effects (Conti 2012, Santos 2003). In this way, ACE2 counterbalances the actions of ACE.
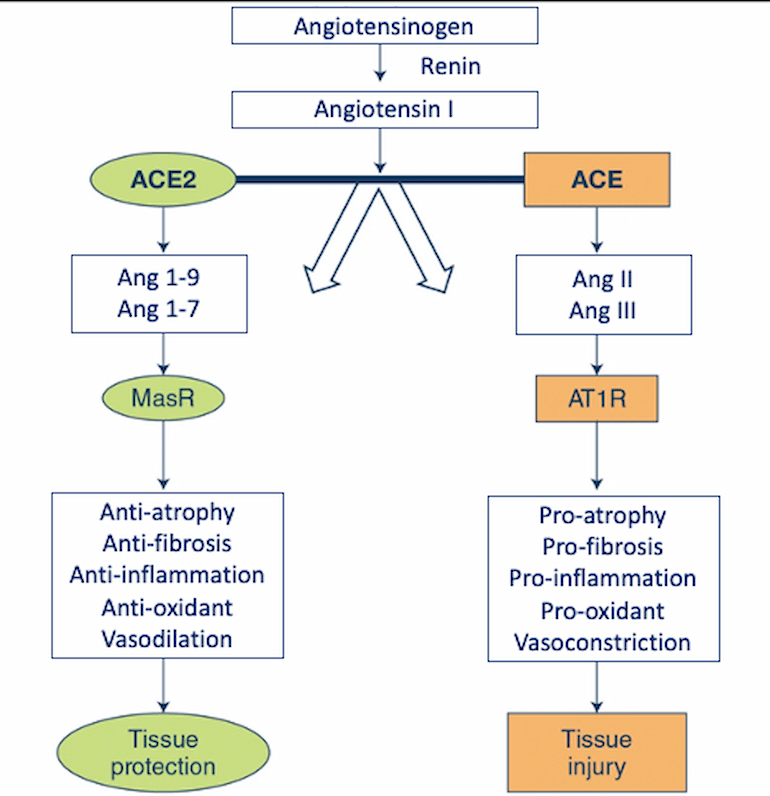
Figure 1
ACE and ACE2 pathways emphasizing opposing downstream effects. Abbreviations: ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; AT1R, angiotensin type 1 receptor; MasR, Mas receptor. (Modified from Zhang 2020).
What is the Tissue and Cell Distribution of ACE2 Expression?
Early reports of COVID-19 manifestations in different organ systems (respiratory, cardiac, GI) have been attributed to gene expression of ACE2 in different organs. However, the distribution of expression of ACE2 is uncertain. ACE2 mRNA has, in fact, been detected in many different organs (lung, heart, GI tract), and at least one immunohistochemistry study confirmed the presence of ACE2 in airway epithelial cells and enterocytes (Hamming 2004). While this pattern may explain pulmonary and GI manifestations of SARS, ACE2 mRNA and protein distribution in different organs (kidney, testis, endothelium) does not match the clinical manifestation of SARS and COVID-19. There is clear recognition that ACE2 gene expression alone cannot predict organ susceptibility to a viral infection. In fact, there are multiple published (Zou 2020, Qi 2020) and ongoing efforts (Travaglini 2020, Zhang 2020, Zhao 2020, Lukassen 2020) to further characterize the expression of ACE2, possible co-receptors, and associated virus-processing genes in different cell types throughout the body.
What Was Known About the Relationship Between ACE, ACE2 and Lung Injury Before COVID-19?
The role of ACE and angiotensin II activity in potentiating lung injury and ARDS has been investigated previously, though their distinct role remains ill-defined. Some have posited that ACE activity predominates when ACE2 expression is attenuated by epithelial injury, such as in ARDS, since components of the ACE pathways, including angiotensin II and activation of its receptor promote ARDS pathogenesis. As such, attempts have been made to explore the balance between ACE and ACE2 activity in ARDS models. When ACE2 knock-out mice were infected with H7H9 influenza, researchers found increased severity of lung injury and higher viral titers (Yang 2015). In an animal model of ventilator-induced lung injury, applying mechanical stretch to human pulmonary epithelial cells resulted in increased ACE expression and increased markers of tissue remodeling and fibrosis (Zhang 2015). In humans, bronchoalveolar lavage fluid from seven patients with ARDS had elevated ACE levels in comparison to normal controls and patients with other pulmonary diseases (Idell 1987). Small studies in humans have demonstrated increased ratios of Ang 1-9/Ang I and Ang 1-7/Ang I in ARDS survivors, suggesting increased ACE2 activity (Reddy 2019). These experiments suggest that ACE2 plays a role in protecting from lung injury.
Others have tried to increase ACE2 activity for therapeutic effect. For example, in 2005, Imai et al. demonstrated that increasing ACE2 levels by blocking the renin-angiotensin pathway or providing recombinant ACE2 in mice protected from acute lung injury (Imai 2005). Furthermore, when rats with LPS-induced ARDS were treated with an angiotensin receptor blocker, ACE2 and Ang 1-7 levels were significantly higher, and lung injury was reduced (Wösten-van asperen 2011).
In humans, Khan et al. tested a recombinant human ACE2 infusion in a phase II trial (Khan 2017). Here, 44 patients with ARDS were randomized to receive recombinant human ACE2 or placebo. After receiving study drug, angiotensin II levels decreased, and Ang 1-7 levels increased. Interestingly, those receiving ACE2 also experienced a decline in respiratory mechanics. However, the study was not powered to detect clinical outcomes and there were baseline imbalances between the control and intervention arms. A phase three trial has not been conducted.
What is the Role of ACE2 in SARS-CoV Infection?
After the outbreak of SARS in 2002-2003, evidence emerged that ACE2 is a receptor for SARS-CoV infection. There is substantial evidence that SARS-CoV binds ACE2 in vitro (Li 2003). Mice expressing transgenic human ACE2 were susceptible to SARS-CoV, consistent with ACE2 sufficiency for this infection. Furthermore, ACE2 knockout mice are relatively resistant to SARS-CoV infection, suggesting that ACE2 is critical for SARS-CoV infection in vivo (Kuba 2005). However, even at the same time that evidence for ACE2 was growing, evidence began to emerge that alternative receptors could serve as portals for SARS-CoV infection (Jeffers 2004). While many now accept as dogma that ACE2 plays a crucial role in SARS-CoV infection, we consider this an open question that requires additional investigation with modern experimental tools.
Is ACE2 the Receptor for SARS-CoV-2?
The 18 years of literature on the role of ACE2 in SARS-CoV infection (Zhang 2020), and the sequence similarity between SARS-CoV and SARS-CoV-2, have led to the logical hypothesis that ACE2 is crucial for SARS-CoV-2 as well. While early reports appear to support this hypothesis, initial reports should be interpreted with great caution. Two early studies have demonstrated that ACE2 expression in several in vitro cell line infection models was sufficient for SARS-CoV-2 infection (Zhou 2020, Hoffmann 2020). However, there is no organism-level genetic proof that SARS-CoV-2 infection requires ACE2.
Furthermore, SARS-CoV-2 spike (S) protein, unlike the SARS-CoV S protein, has a putative furin-like protease cleavage site (Coutard 2020). This difference in sequence between SARS-CoV and SARS-CoV-2 has several important implications. First, this furin-like cleavage site may allow many different proteases to process the S protein and facilitate viral infection of a cell. Second, the furin-like cleavage site in the SARS-CoV-2 S protein further raises the possibility that SARS-CoV-2 can enter the cell in an ACE2-independent manner.
Implications for Patients Receiving ACEi, ARBs or ANRis
Given the COVID-19 disease-modifying potential of manipulating the RAAS pathway and the data demonstrating worse outcomes for patients with cardiovascular disease, there has been heightened interest in the role of ACE-i, ARBs or ARNis. However, at this time, to our knowledge, there are no published data documenting the rates of ACE-i, ARB or ARNi use in COVID-19 patients. Therefore, the concern that these medications may be contributing to susceptibility or severity of disease is only speculative based on the frequency with which these medications are prescribed for the comorbidities that seem to be frequent in COVID-19 patients.
Regardless, the concern about these therapies has taken hold in the lay press and scientific community. However, in light of evidence above showing that increased ACE2 levels may attenuate lung injury, and the uncertainty surrounding ACE2’s role in SARS-CoV-2 infection, this is difficult to reconcile. Furthermore, Patel and Verma pointed out that the effect of chronic ACEi/ARB therapy on expression of ACE2 in the pulmonary epithelium is unclear (Patel 2020). One could possibly argue that upregulation of ACE2 in the setting of chronic ARB/ACEi therapy could actually be protective against more severe COVID-19 disease based on the data in ARDS discussed above.
Certain pharmacologic properties of ACEis, ARBs and ARNis also need to be taken into account. In particular, ACEis differ in their interaction with the ACE enzyme, which produces differences in duration of action, potency and may cause effects beyond solely enzyme inhibition (Furberg 2001, Hanif 2010). These non-ACEi effects are mediated by the sulfhydryl-, carboxyl- or phosphinic moieties on the side chains. For example, captopril is known to have greater venodilating properties than other ACEis (Dr. Randall Zusman, personal communication, 3/28/2020; Capewell 1989). Angiotensin receptor-neprilysin inhibitors also have complex effects on the RAAS system, as well as bradykinin pathway (also impacted by ACEis). The interplay between these medications, the RAAS system and potential ACE2 effects should not be simplified in proposing that there is a risk for all patients on any of these agents.
Multiple professional societies have independently promulgated advice to continue these therapeutics. The European Society of Cardiology has explicitly stated that there no evidence to suggest that ACE-I or ARB therapy is harmful or potentiates the severity of COVID-19 disease. They strongly recommend patients continue treatment as prescribed by their physicians. A similar statement followed jointly from the American Heart Association, the Heart Failure Society of America, and the American College of Cardiology, suggesting not to discontinue any of these therapeutics where they are used for hypertension, heart failure, and ischemic heart disease according to prevailing guidelines. Furthermore, twelve other professional societies have issued similar recommendations.
Conclusions
Consolidating what is known about ACE2, ACE/ACE2 balance in lung injury, SARS-CoV and SARS-CoV-2 coronavirus, it is clear that there is much to learn. We can state with some confidence that elevated ACE2 levels may be protective or attenuate lung injury. Prior data on SARS-CoV and ACE2 do prompt curiosity about the impact of changes in ACE2 level on susceptibility to SARS-CoV-2, but the exact mechanism for viral entry and detailed knowledge about ACE2 expression in the pulmonary epithelium and other tissues is needed. Moreover, we should be hesitant to extrapolate data from SARS-CoV viral mechanisms to SARS-CoV-2, and emphasize further caution before applying this to clinical decision making. Clinicians should continue to observe existing cardiovascular guideline recommendations for ACEi, ARB, and ARNi, with treatment continuation and cessation decisions individualized in part based on a patient’s ambient hemodynamics. Taken together, researchers and clinicians should continue to explore alternative hypotheses for the elevated morbidity and mortality seen in patients with cardiovascular disease and SARS-CoV-2 infection.
References
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:
a single-centered, retrospective, observational study. Lancet Respir Med 2020; published online Feb 24. https://doi.org/10.1016/S2213- 2600(20)30079-5. - Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China [published online ahead of print, 2020 Feb 7]. JAMA. 2020;e201585.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30;:]. Lancet. 2020;395(10223):497–506.
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; published online Feb 28. DOI:10.1056/NEJMoa2002032.
- Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China [published online ahead of print, 2020 Mar 13]. JAMA Intern Med. 2020;e200994.
- Arentz M, Yim E, Klaff L, et al. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State [published online ahead of print, 2020 Mar 19]. JAMA. 2020;e204326.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published online ahead of print, 2020 Mar 11]. Lancet. 2020;S0140-6736(20)30566-3.
- Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China [published online ahead of print, 2020 Mar 25]. JAMA Cardiol. 2020;10.1001/jamacardio.2020.0950.
- Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China [published online ahead of print, 2020 Mar 11]. Clin Res Cardiol. 2020;10.1007/s00392-020-01626-9.
- Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? [published online ahead of print, 2020 Mar 11]. Lancet Respir Med. 2020;S2213-2600(20)30116-8.
- Zhang H, Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit Care. 2017;21(1):305. Published 2017 Dec 13. doi:10.1186/s13054-017-1882-z
- Santos RA, Simoes e Silva AC, Maric C, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U. S. A. 2003;100(14):8258–8263. doi:10.1073/pnas.1432869100
- Conti S, Cassis P, Benigni A. Aging and the renin-angiotensin system. Hypertension. 2012;60(4):878-83.
- Yang P, Gu H, Zhao Z, et al. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep. 2014;4:7027.
- Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China [published online ahead of print, 2020 Mar 25]. JAMA Cardiol. 2020;10.1001/jamacardio.2020.0950.
- Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penniger JM (2005) Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436:112–116.
- Wösten-van asperen RM, Lutter R, Specht PA, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol. 2011;225(4):618-27.
- Zhang R, Pan Y, Fanelli V, et al. Mechanical Stress and the Induction of Lung Fibrosis via the Midkine Signaling Pathway. Am J Respir Crit Care Med. 2015;192(3):315-23.
- Idell S, Kueppers F, Lippmann M, et al. Angiotensin converting enzyme in bronchoalveolar lavage in ARDS. Chest 1987; 91: 52–56.
- Reddy R, Asante I, Liu S, et al. Circulating angiotensin peptides levels in Acute Respiratory Distress Syndrome correlate with clinical outcomes: A pilot study. PLoS ONE. 2019;14(3):e0213096.
- Khan A, Benthin C, Zeno B, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21(1):234.
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, Van goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631-7.
- Travaglini KJ, Nabhan AN, Penland L, et al. 2020. A molecular cell atlas of the human lung from single cell RNA sequencing. bioRxiv doi: 10.1101/742320.
- Zhang H, Kang Z, Gong H et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv doi: 10.1101/2020.01.30.927806.
- Zhao Y, Zhao Z, Zhou Y, Ma Y, Zuo W. 2020. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv doi: 10.1101/2020.01.26.919985.
- Lukassen S, Chua RL, Trefzer T, et al. 2020. SARS-CoV-2 receptor ACE2 and TMPRSS2 are predominantly expressed in a transient secretory cell type in subsegmental bronchial branches. bioRxiv doi: 10.1101/2020.03.13.991455.
- Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020.
- Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses [published online ahead of print, 2020 Mar 18]. Biochem Biophys Res Commun. 2020;S0006-291X(20)30523-4.
- Li, W., Moore, M., Vasilieva, N. et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454 (2003). https://doi.org/10.1038/nature02145
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus– induced lung injury. Nat Med 11:875–879.
- Jeffers SA, Tusell SM, Gillim-Ross L, et al. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci U. S. A. 2004;101(44):15748–15753.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor [published online ahead of print, 2020 Mar 4]. Cell. 2020;S0092-8674(20)30229-4.
- Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742.
- Yang XH, Deng W, Tong Z, et al. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med. 2007;57(5):450–459.
- P. Zhou, X.-L. Yang, X.-G. Wang, B. Hu, L. Zhang, W. Zhang, H.-R. Si, Y. Zhu, B. Li, C.-L. Huang, H.-D. Chen, J. Chen, Y. Luo, H. Guo, R.-D. Jiang, M.-Q. Liu, Y. Chen, X.-R. Shen, X. Wang, X.-S. Zheng, K. Zhao, Q.-J. Chen, F. Deng, L.-L. Liu, B. Yan, F.-X. Zhan, Y.-Y. Wang, G.-F. Xiao, Z.-L. Shi, A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579, 270–273 (2020).
- M. Hoffmann, H. Kleine-Weber, S. Schroeder, N. Krüger, T. Herrler, S. Erichsen, T. S. Schiergens, G. Herrler, N.-H. Wu, A. Nitsche, M. A. Müller, C. Drosten, S. Pöhlmann, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell (2020), doi:10.1016/j.cell.2020.02.052.
- B. Coutard, C. Valle, X. de Lamballerie, B. Canard, N. G. Seidah, E. Decroly, The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 176, 104742 (2020).
- Patel AB, Verma A. COVID-19 and Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers: What Is the Evidence? [published online ahead of print, 2020 Mar 24]. JAMA. 2020;10.1001/jama.2020.4812.
- Furberg CD, Pitt B. Are all angiotensin-converting enzyme inhibitors interchangeable?. J Am Coll Cardiol. 2001;37(5):1456-60.
- Hanif K, Bid HK, Konwar R. Reinventing the ACE inhibitors: some old and new implications of ACE inhibition. Hypertens Res. 2010;33(1):11-21.
- Capewell S, Taverner D, Hannan WJ, Muir AL. Acute and chronic arterial and venous effects of captopril in congestive cardiac failure. BMJ. 1989;299(6705):942-5.
View all COVID-19 updates
Learn about research in the Division of Pulmonary and Critical Care Medicine