BRD4 Inhibitors May Be Effective Against a Subset of Head and Neck Squamous Cell Carcinomas
Key findings
- Dysfunction of the Hippo-YAP pathway, which plays critical roles in cell signaling and proliferation, is common in head and neck squamous cell carcinoma (HNSCC) and other cancers
- This study investigated the role of the YAP1/BRD4 axis in the tumorigenesis of HNSCC and the potential to find therapeutic targets
- Mutation of FAT1, a core component of the Hippo pathway, led to activation of YAP1, which then coupled with BRD4 to maintain an oncogenic chromatin state
- FAT1-mutant human HNSCC cells were sensitive to BRD4 inhibitor therapy, and PD-L1, the target of several FDA-approved checkpoint inhibitor immunotherapies, was a downstream target regulated by YAP1 (and BRD4)
- In patients with FAT1-mutated HNSCC, it may be possible to enhance anticancer immunity by blocking PD-L1 with a BRD4 inhibitor
Core components of the Hippo pathway are known to be deregulated in human squamous cell carcinoma, including head and neck squamous cell carcinoma (HNSCC). Three of these are:
Subscribe to the latest updates from Otolaryngology Advances in Motion
- The upstream negative regulator FAT1 (FAT atypical cadherin 1)
- The downstream transcriptional co-activators YAP1 (Yes-associated protein 1) and TAZ (transcriptional coactivator with PDZ-binding motif, also called WWTR1)
FAT1-mutated SCCs undergo epithelial-to-mesenchymal transition, and FAT1 loss of function promotes YAP1 nuclear translocation and activation. In turn, YAP1 amplification promotes tumor initiation, malignancy and metastasis. Furthermore, it was recently reported in Clinical Cancer Research that YAP1 mediates resistance to trametinib in human HNSCC, and other studies have linked YAP1 to drug resistance in additional cancers.
Inhibition of BRD4 (bromodomain-containing protein 4) can blunt YAP/TAZ pro-tumorigenic activity, cause regression of established neoplasms and reverse drug resistance in breast cancer. BRD4 is an epigenetic regulator that recognizes and binds the acetylated lysine residues in histone, and it's become well known for its role in transcription activation of several prominent oncogenes.
Srinivas Vinod Saladi, PhD, assistant scientist at the Mike Toth Head and Neck Cancer Research Center at Mass Eye and Ear, and colleagues explored the coordinated role of the YAP1/BRD4 axis in HNSCC. In Cell Reports they describe the potential to selectively target YAP1 through BRD4 inhibition in patients with FAT1-mutated HNSCC.
Principal Findings
In laboratory studies, the research team found that:
- FAT1 depletion led to activation of YAP1, which then coupled with BRD4 to maintain an oncogenic chromatin state
- While FAT1-mutant human HNSCC cells were sensitive to multiple cancer therapeutics, the best results came with JQ1, a first-in-class BRD4 inhibitor being tested in a variety of cancer trials
- ATAD2, which contributes to cancer cell proliferation, invasion and migration in multiple tumor types, proved to be involved in YAP1 transcriptional activation and was a direct downstream target of BRD4 inhibition
- PD-L1, the target of several FDA-approved checkpoint inhibitor immunotherapies, was also a downstream target regulated by BRD4
- Expression of PD-L1 was higher in HNSCC primary tumors than normal tissues and presumably helps the tumors evade intrinsic immune response
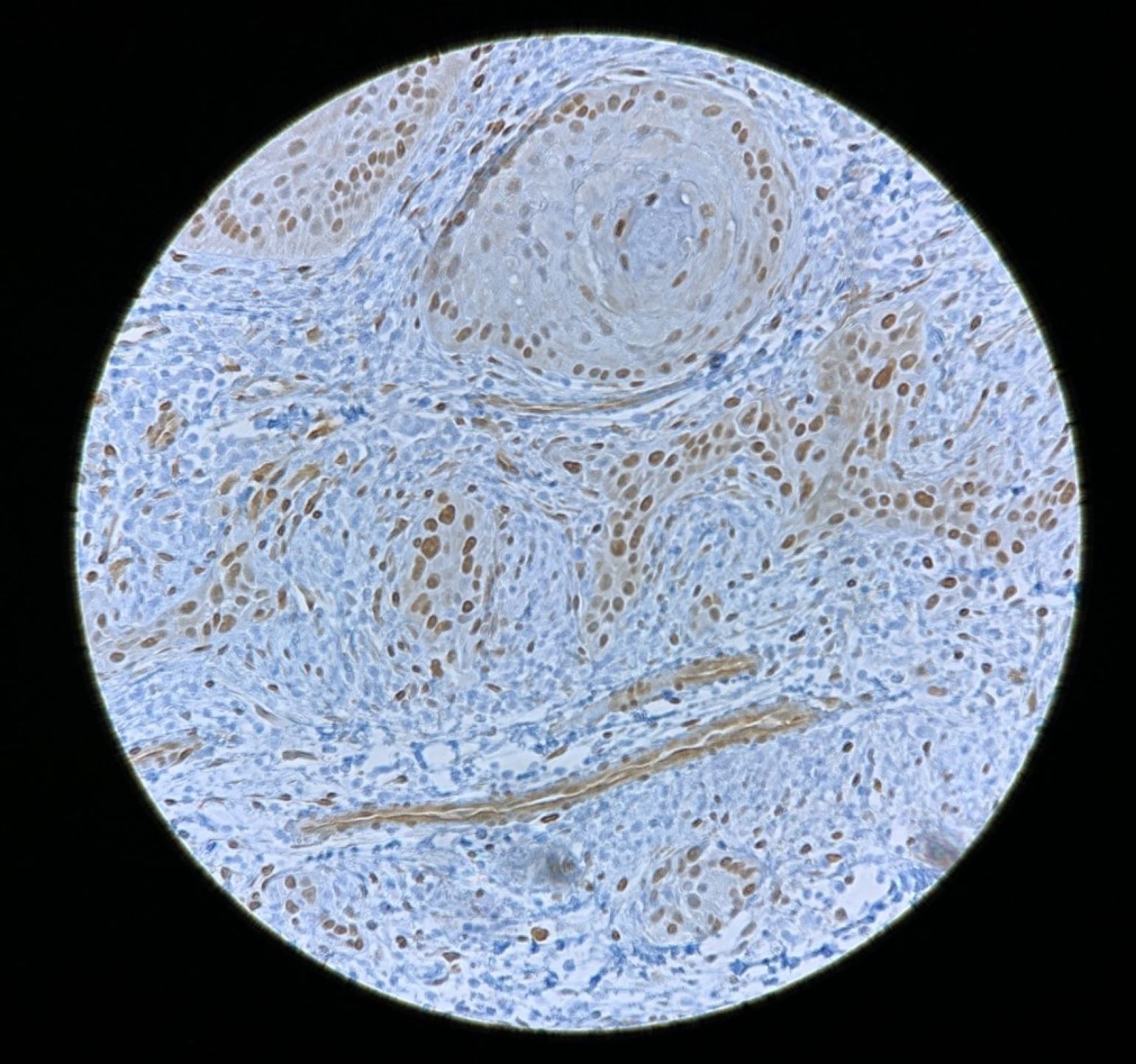
Figure 1
YAP1 staining in head and neck squamous cell carcinoma.
Toward Clinical Translation
In patients with FAT1-mutated HNSCC, it may be possible to enhance anticancer immunity by blocking PD-L1 through BRD4 inhibition with JQ1 or the newer generation BRD4 inhibitors. The next step will be to see whether this strategy is translatable to patients by utilizing patient-derived organoids and xenografts.
The findings of this study might extend beyond HNSCC, so the FAT1–YAP1/BRD4 axis deserves careful characterization in other cancer types.
view original journal article Subscription may be required
Learn more about Mass Eye and Ear/Mass General Brigham
Refer a patient to Mass Eye and Ear/Mass General Brigham