Gene Therapy Reverses Degeneration of the Retinal Pigment Epithelium in Preclinical Models
Key findings
- Although oxygen is necessary for the retina to function, a hyperoxic environment can be harmful. A potential therapeutic strategy for retinitis pigmentosa is to prevent oxidative damage
- In this mouse model of retinitis pigmentosa, researchers observed that retinal pigment epithelium (RPE) cells and nearby retina cells died from oxidative stress
- Previous research in mice showed that gene therapy with Nrf2, a transcription factor that regulates antioxidant genes, led to longer survival of cones and longer retention of vision
- Using adeno-associated viral vector (AAV) gene therapy, researchers overexpressed Nrf2 in mouse RPE and were able to upregulate oxidative stress defense in the RPE and prevent RPE loss. This also protected overlying cone photoreceptors and improved vision
- Based on these findings, Nrf2 AAV gene therapy has potential implications not only for the treatment of retinitis pigmentosa, but also for age-related macular degeneration, as oxidative stress is thought to be one of its central disease mechanisms
Oxygen is both a necessity and a danger to living tissues. That is the case in the eye, where the retina is doing the work of translating incoming photons into vision. The retina's oxygen demand is so high that it has its own specialized vasculature—the choroid—to deliver a higher blood flow than any other circulatory bed in the body. Cells in the retina live on a finely balanced edge that can turn deadly quickly.
Subscribe to the latest updates from Ophthalmology Advances in Motion
Take for instance a disease like retinitis pigmentosa, where rod photoreceptors get sick and die. Although we can lose some rods while still retaining vision, losing these rods also means there are fewer cells to consume oxygen from the choroid. The remaining retina is exposed to a higher level of oxygenation and oxidative stress. This starts a cascade, leading to the loss of other cell types, such as cone photoreceptors, whose death leads to more devastating central and high acuity vision loss. These findings have led researchers to propose fighting oxidative stress as a way to stop retinitis pigmentosa.
A New Target to Prevent Vision Loss
David M. Wu, MD, PhD, a retina specialist and assistant scientist at Mass Eye and Ear/Mass General Department of Ophthalmology, and assistant professor of Ophthalmology at Harvard Medical School, and Constance Cepko, PhD, a virologist and professor of Ophthalmology and Bullard professor of genetics and neuroscience at Harvard Medical School, led a team examining this phenomenon from a new angle in a mouse model of retinitis pigmentosa. The study, published in the Journal of Clinical Investigation, investigated the retinal pigment epithelium (RPE), a layer of cells that nurtures the photoreceptors.
Located between the choroid and the retina, the RPE is directly adjacent to the high oxygenation from choroidal blood flow. Dr. Wu's team found that as the rods degenerate in oxidative stress, the RPE cells lose the regular structure. RPE loss is a key cause for vision loss in an even more common retinal disease, age-related macular degeneration (AMD). The researchers decided to fortify the RPE from oxidative damage to see if they could protect it and the retina in this disease model.
The researchers modified an adeno-associated virus (AAV)—the same type of vector used in human gene therapy applications—adapting it so that they could overexpress a molecule called Nrf2 selectively in RPE cells. Nrf2 is a transcription factor that turns on the cell's defenses to oxidative stress and inflammation. Nrf2 signaling declines with age, and some have theorized that this is one of the mechanisms by which degenerative changes secondary to aging occur. By overexpressing Nrf2, they sought to reverse such changes, harnessing and upregulating multiple endogenous protective pathways in the cell.
The researchers found that RPE cells receiving AAV-Nrf2 gene therapy structurally appeared as if they had no stress from hyperoxia at all. Furthermore, even though the expression of the AAV was localized to the RPE, the protection extended beyond the RPE to the overlying cone photoreceptors that are so vital for central vision.
In untreated retinitis pigmentosa mice, the regularly spaced array of cone photoreceptors was disrupted and filled with craters devoid of visual cells. However, in eyes whose RPE were rescued by Nrf2, the intact RPE layer kept this cone layer intact. Dr. Wu's team assessed the vision of rescued mice and found that they had better vision.
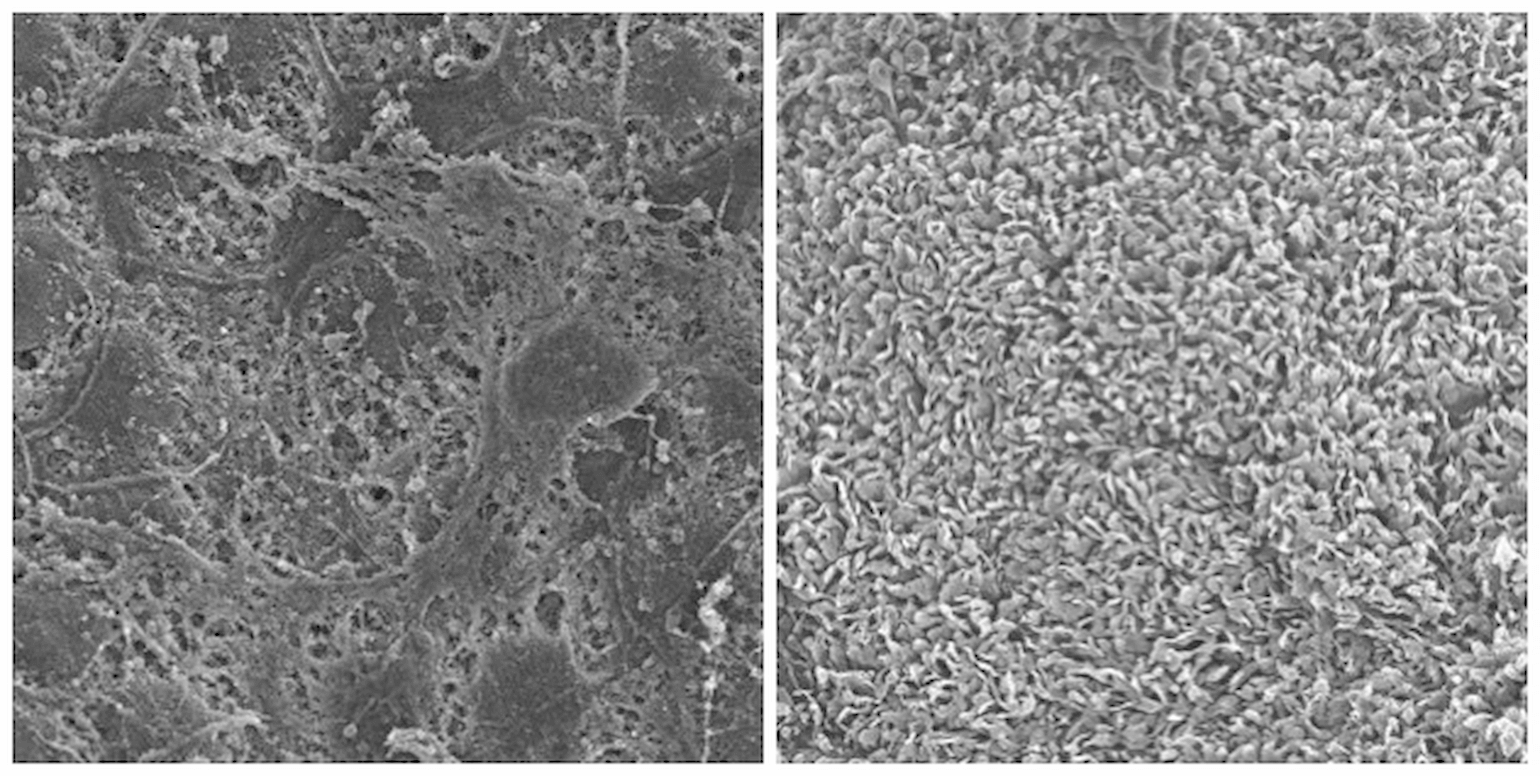
Figure 1
Scanning electron microscopy of mouse RPE flat-mount with RP (left panel), or RPE flat-mount with RP rescued by AAV-Nrf2 (right panel). The RPE cells on the left are largely barren of any microvilli. The contralateral eye of the same mouse received AAV-Nrf2. The RPE are indistinguishable from wild type mice and have a full complement of microvilli processes that interdigitate with the photoreceptors.
Potential Applications to AMD
The researchers then employed transcriptome profiling, a way to take a snapshot of all the gene activity in a cell, including how it responds to its environment. They found that key oxidative defense pathways were decreased in the RPE of mice that had retinitis pigmentosa, but were increased in those rescued by Nrf2 overexpression. Several of these pathways have been implicated in the progression of AMD as well, and RPE degeneration plays a central role in the pathogenesis of AMD.
Thus, the researchers found that the RPE plays a potential role in the degeneration of the retina seen in retinitis pigmentosa and that Nrf2 gene therapy to the RPE could prevent both anatomical and functional damage.
These findings have potential implications for not only retinitis pigmentosa, but also for AMD, where RPE loss is associated with the loss of photoreceptors and vision. Because Nrf2 reverses RPE loss in retinitis pigmentosa and restores many of the pathways thought to be important in RPE death, it is now under consideration as a gene therapy approach for AMD.
view original journal article Subscription may be required
Learn more about the Department of Ophthalmology at Mass Eye and Ear/Mass General
Refer a patient to Mass Eye and Ear/Mass General