Hospitals Urged to Adopt High-Sensitivity Cardiac Troponin Assays
Key findings
- High-sensitivity cardiac troponin (hs-cTn) assays, now approved by the FDA, differ considerably from established cardiac troponin tests
- The 4th Universal Definition of Myocardial Infarction consensus panel recommends transition to hs-cTn testing in all hospital services
- A central theme of the panel's report is the need for clinical laboratory staff to collaborate with other services to decide how hs-cTn assays can best be implemented in different settings, such as the emergency department and inpatient services
- Well before an institution converts to hs-cTn testing, extensive education should be provided to all affected services
High-sensitivity cardiac troponin (hs-cTn) assays were recently approved by the U.S. Food and Drug Administration. These assays differ considerably from established cardiac troponin tests, and there is variability in cutoff values, sensitivity, specificity and methods of interpretation.
Subscribe to the latest updates from Cardiovascular Advances in Motion
The American College of Cardiology recently convened an expert panel to develop a consensus about what medical systems should consider before implementing hs-cTn assays. James Louis Januzzi, MD, director of the Dennis and Marilyn Barry Fellowship in Cardiology Research at Massachusetts General Hospital, served as the first author of the panel's report, which appears in the Journal of the American College of Cardiology.
The Expert Panel recommends a transition to hs-cTn for all hospital services. Key themes of the panel's report, summarized in the illustration below, are the need for collaborative preparation for the transition and the need for extensive education of hospital personnel:
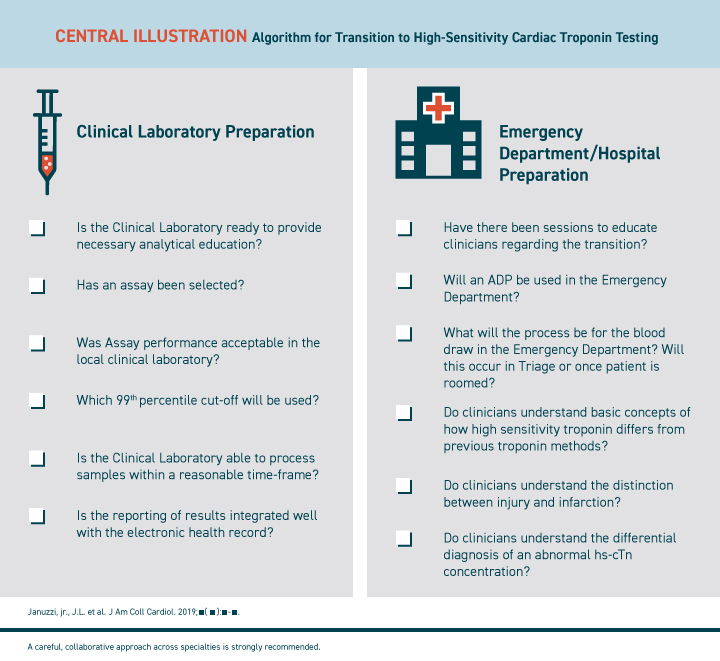
Central Illustration: ALGORITHM FOR TRANSITION TO HIGH-SENSITIVITY CARDIAC TROPONIN TESTING
The panel recommends using a collaborative, cross-specialty approach to adapting hs-cTn testing.
The Need for Collaborative Preparation
In consultation with other services, clinical laboratory staff should first decide which hs-cTn assay best fits local needs. Decisions about which rule-out and diagnostic strategies to use in various environments should involve leaders from the emergency department, cardiology, hospital medicine, surgery, anesthesia, critical care, nursing and other important specialties as appropriate.
Testing in the Emergency Department
The expert panel recommends excluding myocardial infarction (MI) using a rapid algorithm for hs-cTn (e.g., baseline plus a second sample one, two or three hours later), along with a validated risk score such as EDACS or HEART, to identify patients suitable for early discharge. Serial testing is less likely to miss MI among early presenters and uses a change value that is less susceptible to assay imprecision.
- For patients presenting >3 hours from symptom onset, a 0/1-hour triage algorithm can provide acceptable sensitivity and negative predictive values
- For patients presenting >3 hours from symptom onset who have a low-risk clinical presentation and a very low initial hs-cTn concentration, the single-test approach is reasonable
- For patients presenting <3 hours from symptom onset, evidence suggests that the single-test approach might have lower sensitivity, so serial testing is always recommended in that group
The expert panel agrees with the Universal Definition of MI and suggests using sex-based 99th-percentile upper reference limits. It provides recommended values for significant changes of hs-cTn to be nested within the strategies used for evaluation of acute MI.
Testing Inpatients
For inpatient testing of acute chest discomfort or other signs of myocardial ischemia, the expert panel advises using the three-hour approach to hs-cTn testing. The rapid response for inpatients can result in early false-negative results and difficulties in seeing a delayed falling pattern.
Compared with the emergency department setting, substantially more inpatients are likely to have significant, acute comorbidities that can cause myocardial injury. The expert panel provides guidance about how to approach short-term hs-cTn changes, how to differentiate long-term hs-cTn elevations and how to distinguish between coronary and noncoronary myocardial injury and type 1 and type 2 MI.
Stable Outpatients
There are insufficient data to inform the use of hs-cTn testing for stable outpatients, the report's authors conclude, and they recommend against it, at least for now.
The Need for Education
Before an institution converts to hs-cTn assays, gradual, phased education must be provided to all affected services, the expert panel emphasizes. The process should take at least several weeks to months, to allow for preparation in the laboratory and provide time for busy clinicians to familiarize themselves with the information needed to make the transition.
The educational program should feature didactic lectures, enduring materials, including electronic and print media and laminated cards detailing important information, and frequent reminders before launch. Key topics should be how hs-cTn assays differ from conventional troponin assays, how to interpret hs-cTn concentrations and strategies for responding to confusing results.
Once hs-cTn testing is implemented, the institution should monitor patterns of hs-cTn ordering, interpretation and patient treatment. Outcomes should be evaluated and any barriers to hs-cTn testing should be addressed.
view original journal article Subscription may be required
Learn more about the Corrigan Minehan Heart Center
Refer a patient to the Corrigan Minehan Heart Center