Modified Paclitaxel Plus Daily Radiation Helps Noncystectomy Candidates
Key findings
- Daily radiation combined with a modified paclitaxel regimen was effective for patients with muscle-invasive urothelial bladder cancer who were unfit for radical cystectomy or platinum-based radiosensitizing chemotherapy
- In patients whose tumors overexpressed HER2, the addition of trastuzumab to the regimen appeared to result in comparable efficacy and toxicity
- A majority of patients successfully completed therapy, and a majority of evaluable patients manifested complete response to treatment
Subscribe to the latest updates from Urology Advances in Motion
For patients with muscle-invasive urothelial carcinoma (UC), bladder preservation strategies such as platinum-based chemotherapy, hyperfractionated radiation and selective cystectomy are proven alternatives to radical cystectomy. However, many patients with UC who are not candidates for radical cystectomy have comorbidities that are absolute or relative barriers to these treatments.
Researchers at Massachusetts General Hospital have found that a modified non-platinum chemotherapy regimen and once-daily radiation is effective for patients with muscle-invasive UC who are not candidates for cystectomy. The novel strategy was associated with a high completion rate and moderate toxicity. In patients with HER2-positive tumors, a group generally considered to have worse outcomes, the addition of trastuzumab appeared to result in comparable efficacy and toxicity.
Mark Dror Michaelson, MD, PhD, clinical director of The Claire and John Bertucci Center for Genitourinary Cancers, Douglas M. Dahl, MD, chief of the Division of Urologic Oncology, Chin Lee-Wu, MD, PhD, director of Genitourinary Pathology Services, William Shipley, MD, the Andres Soriano distinguished professor of Radiation Oncology, and colleagues report the findings in the International Journal of Radiation Oncology * Biology * Physics.
In a phase 1/2 study, the researchers enrolled 66 patients who had primary UC with muscularis propria invasion and no evidence of metastasis. All patients received paclitaxel 50 mg/m2, given on days 1, 8, 15, 22, 29, 36 and 43. All also received daily fractionated radiation therapy (RT), administered using a shrinking field technique. Twenty of the patients had tumors that overexpressed HER2, and they also received weekly trastuzumab, because such tumors are thought to have inferior outcomes with traditional therapy alone.
The primary endpoint of this study was acute treatment-related adverse events (AEs) that did not resolve to grade 1 within seven days or a reasonable time frame. The protocol-specified AEs were:
- Grade 4 neutropenia or grade 4 febrile neutropenia
- Grade 3 diarrhea, nausea or vomiting
- Grade 3 thrombocytopenia
- Specific grade 3 non-hematologic toxicities: renal, pulmonary, hepatic or neurologic toxicity; rectal or genitourinary bleeding; left ventricular failure
- Grade 2 cardiac toxicity (besides left ventricular failure)
- Inability to complete chemoradiation because of treatment-related toxicity
Protocol-specified AEs were observed in 35% of the HER2-overexpressing patients who received RT + paclitaxel + trastuzumab and 30.4% of the remaining 46 patients who received RT + paclitaxel alone (Table 1). In both groups, the most common grade 3 and grade 4 AEs were gastrointestinal, particularly diarrhea. In the trastuzumab group there was one death, caused by colonic perforation 10 days after treatment completion, which was considered possibly related to treatment.
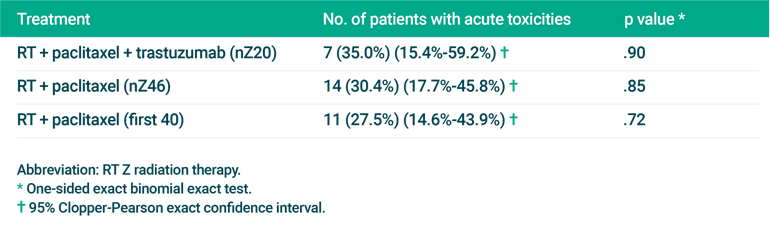
Table 1: Incidence of Protocol-specified Acute Treatment-related Toxicity
The researchers note that, overall, the rate of AEs was similar to that seen in prior studies of bladder preservation therapy.
Treatment completion rate was one of the prespecified secondary endpoints. Sixty percent of patients in the RT + paclitaxel + trastuzumab group and 73.9% of the RT + paclitaxel group completed treatment per protocol.
When analyzed at one year, the complete response rate was 72.2% in the RT + paclitaxel + trastuzumab group and 67.6% in the RT + paclitaxel group.
The researchers conclude that the novel treatment approach is a reasonable strategy for this challenging patient population. They add, though, that because of the small number of patients who received RT + paclitaxel + trastuzumab, no definitive conclusions should be drawn about the efficacy and toxicity of that regimen in this study.
view original journal article Subscription may be required
About the Urologic Oncology program