Pets and COVID-19
The FLARE Four
- Scattered reports have emerged of positive viral testing in household pets, raising concern for human-to-pet transmission or vice versa
- SARS-CoV-2 infection in domesticated animals appears to lead to a mild and limited disease, if any
- The CDC and professional veterinary medicine groups have provided specific advice to pet owners regarding cautious care and interaction with their own animals, should pet owners become sick
- Available evidence does not indicate that domestic animals are playing a major part in the spread of COVID-19, though further study is needed
Many people are wondering...can my pet get COVID-19?
Subscribe to the latest updates from FLARE Advances in Motion
Introduction
The available evidence indicates that SARS-CoV-2 arose in bats and was likely transmitted to pangolins as an intermediate host (May 12th FLARE). Given the apparent ability of the virus to infect an "animal", a question arises as to whether domestic animals may become infected, or even transmit the virus. There have been reports of captive animals contracting SARS-CoV-2 with differing levels of severity, raising suspicion for human-to-animal transmission. Most concerning is the notion that during social distancing, isolation and quarantine at home, pet owners may be exposing their pets to SARS-CoV-2. In tonight’s FLARE, we will explore the available evidence surrounding infection in and susceptibility of common household pets to SARS-CoV-2.
Can Pets Get SARS-CoV-2? Do They Get Sick?
On March 5th, the AP reported that a dog (not a "hound dog", just a dog) in "Hong Kong" tested (weakly) positive for the virus by three separate swabs. This observation was extended by a study of 15 dogs living with SARS-CoV-2-infected humans, revealing two (a 17 year old Pomeranian and a 2.5 year old German Shepherd) to be swab PCR-positive and with positive serological testing. Viral sequencing indicated that the affected dogs harbored virus identical to their human contacts, suggesting they “caught” SARS-CoV-2 from these human contacts. Both animals were quarantined and remained asymptomatic.
The first report of animal infection in the United States came on April 5, 2020 from the Bronx Zoo (NYC), where a 4-year-old Malayan "tiger" named Nadia, her sister Azul, two Amur tigers, and three African lions developed dry cough, wheezing, and loss of appetite. One of the tigers was tested (only one, because sample collection in a tiger requires general anesthesia!) and was positive for SARS-CoV-2. Nadia first began to show signs of illness on March 27th, about 2 weeks after the zoo had been closed to the public. Zoo staff believe the animals contracted the illness from an animal caretaker who was shedding virus but not yet symptomatic. It is not known whether there was any feline-to-feline transmission.
Subsequently, the first American pets to test positive for the virus were reported. On April 22, 2020, the CDC and the AP described two domestic "cats" in New York state who developed cough and rhinorrhea and were thought to have contracted the virus from their owners or neighbors. The cats were from different households in different parts of the state. Furthermore, a pre-print, non-peer reviewed study reported that of 102 cats in Wuhan sampled after the outbreak (as well as 39 prior to the outbreak), 14.7% samples were positive for anti-SARS-CoV-2 antibodies, indicating unexpectedly high rates of infection among domestic cats (Zhang et al., 2020).
For interest, an updated summary of reports of naturally acquired SARS-CoV-2 infections in domestic animals and farmed or captive wildlife is provided in the table below.
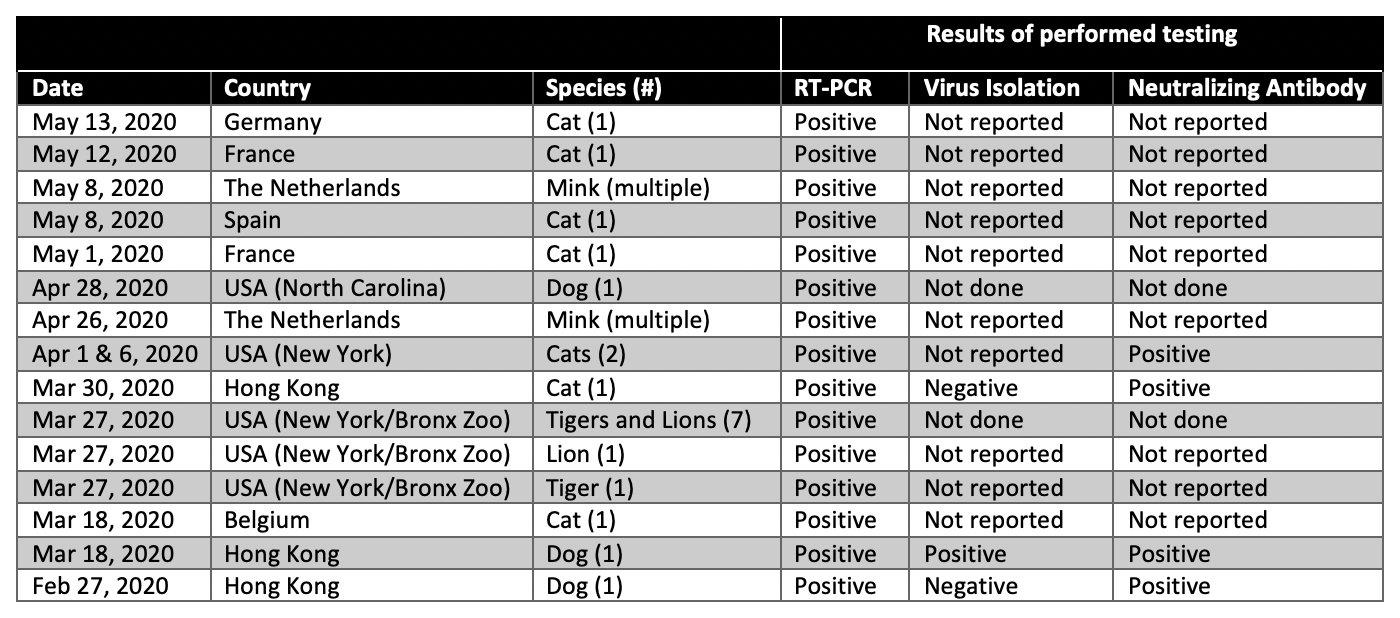
Table
Adapted from and more details available here through the AVMA.
Together, these data are strongly suggestive of human to animal viral transmission but leave unanswered (1) whether transmission in the opposite direction is possible and (2) if house pets are at risk of symptomatic clinical illness attributable to SARS-CoV-2 (Sit et al., 2020).
What Experimental Data Can be Brought to Bear on These Questions?
In Cats?
Halfmann and colleagues evaluated nasal shedding of SARS-CoV-2 and transmissibility via direct contact from 15-18 week old cats inoculated with virus (Halfmann et al., 2020). Three cats were inoculated with virus and co-housed with other cats in three separate pairs. Nasal and rectal swabs were obtained daily from all subjects. In order to evaluate intact virions (rather than RNA presence alone), samples were tested for infectivity in engineered cell lines which support SARS-CoV-2 replication.
By day 3, SARS-CoV-2 was present in nasal swabs from all three of the inoculated cats and persisted until day 5 (1/3) or day 6 (2/3) but was undetectable in any rectal swabs. Interestingly, despite uniformly asymptomatic infection, all inoculated cats developed very high titers of anti-SARS-CoV-2 IgG by day 24, indicating that cats mount an immune response to this viral infection even in the absence of clinical disease. As for transmissibility, the co-housed (ie. non-inoculated) cats all tested positive for infectious virus after five days of cohabitation. Viral shedding from the nose lasted 4-5 days, similar to the inoculated cats. Again, no viable virus was detected from rectal swabs, and no subjects showed any symptoms of illness. The data demonstrate the possibility of transmission between co-located cats but reinforce the lack of clinical significance of this infection.
Shi and colleagues evaluated the susceptibility and transmission potential of multiple animal species to SARS-CoV-2 infection, including cats (Shi et al., 2020). Experiments were performed in subadult cats (aged 6-9 months) and juvenile cats (aged 70-100 days) inoculated with intranasal virus. To evaluate transmissibility, the cages of three inoculated subadult cats were placed next to three un-inoculated subadult cats. The feces and internal organs of the inoculated and exposed cats were subsequently examined for the presence of viral RNA (nasal washings were not collected in this study “because the cats were too aggressive”; though none were evidently driven to "Roar"). Viral RNA was detected in the feces of the inoculated cats and in a single exposed cat. Upon histopathologic examination, there was viral RNA found in the upper respiratory tract of a single exposed cat, though this was not consistent across all pairs of inoculated-exposed cats. The authors offer that this is evidence of respiratory droplet transmission between this single pair of cats.
To assess susceptibility, the authors performed histopathological examinations of cats at 3 and 6 days after inoculation and evaluated for the presence of viral RNA and infectious virus in tissues and organs. On day 3 post-inoculation, viral RNA was present in the upper respiratory tract, lungs and small intestines. On day 6 post-inoculation, viral RNA was not detected in any of the lung samples from either of the animals. Interestingly, the presence of viral RNA did not always correspond to the presence of live virus, highlighting the importance of viral culture. Levels of viral RNA and live virus were lower by day 6. Juvenile cats (70-100 days of age) had higher titers of infectious virus than subadult cats. Importantly, there is no reporting of clinical signs or symptoms of the animals inoculated or exposed to the infection, although juvenile cats had inflammatory lesions in their nasal and tracheal epithelia. Finally, similar to the study by Halfmann and colleagues, antibodies against SARS-CoV-2 were detected in all virus-inoculated subadult cats.
In "Dogs"?
Shi and colleagues also investigated the susceptibility of 3-month old beagles to SARS-CoV-2 infection and transmissibility. After intranasal inoculation (day 0), five beagles were housed with two uninoculated beagles in a room. The authors collected oropharyngeal and rectal swabs between day 2 and day 14 for RNA detection and virus titration in Vero E6 cells. Importantly, infectious virus was not isolated from a single swab from any dog. One dog with a rectal swab positive for RNA did not have any viral RNA detected in any tissues examined upon euthanasia. No information was collected about clinical signs or symptoms of infection. Finally, two virus-inoculated dogs had positive antibodies when tested on day 14, though neither of the uninoculated beagles became seropositive. The authors conclude that “these results indicate that dogs have low susceptibility to SARS-CoV-2”.
In summary, this single study in dogs suggests that they may be susceptible to infection, but are likely to be asymptomatic. Transmissibility from dog-to-dog is not supported by the limited evidence, and dogs appear to be less susceptible than cats (Shi et al. 2020).
In Hamsters?
Hamsters are used as a model of disease and the golden (or Syrian) hamster has been evaluated as a possible animal model for SARS-CoV-2 infection. Hamsters harbor an ACE2 receptor recognizable by the SARS-CoV spike protein and infectivity has been demonstrated in hamster models of SARS but, notably, not of MERS (which utilizes a distinct cell surface receptor; Roberts et al., 2005). Of note, mouse ACE2 does not bind efficiently to SARS-CoV-2 spike protein.
Sia and colleagues inoculated hamsters with SARS-CoV-2 and demonstrated viral antigen in nasal mucosa and the bronchial tree as well lung consolidations suggestive of infection (Sia et al., 2020). Healthy animals placed in cages with infected animals were also shown to become ill via direct contact and aerosols with infectious virus were detected 1 day after cohabitation. When well animals were transferred to the previously soiled cages of sick hamsters, the investigators observed transmission as well, albeit less efficiently than by direct contact (only a third of animals had detectable viral RNA in fecal samples (Sia et al., 2020). These data overall suggest that hamsters are susceptible to SARS-CoV-2 infection and that, in order of transmission efficiency, virus may be passed by aerosolization, direct contact, or fomite transmission.
In Other Animals?
Shi and colleagues also studied viral replication of SARS-CoV-2 in other domestic and livestock animal species after experimental intranasal inoculation (Shi et al., 2020). They found efficient replication of virus in ferrets and cats, minimal replication in dogs, and no replication in pigs, chickens, or ducks. The authors did not seek to test a "fox".
Ferrets are frequently used as a model of human respiratory viral infection due to their similar lung physiology, distribution of cellular receptors, susceptibility to human respiratory viruses, and clinical signs and symptoms of infection (Belser et al., 2016). Shi and colleagues detected SARS-CoV-2 (via viral RNA quantification and virus titration in Vero E6 cells) in the nasal turbinate, soft palate, and tonsils of four inoculated ferrets, but not in any of the other organs tested. Six additional ferrets were inoculated and monitored for signs of illness for two weeks. Viral RNA was detected in nasal swabs on days 2 through 8 in all six, and all developed an antibody response. Two ferrets developed respiratory symptoms on days 10 and 12 after inoculation. The ferrets were euthanized on day 13, at which time pathologic evaluation of the lungs revealed lymphoplasmacytic perivasculitis and vasculitis, increased numbers of type II pneumocytes, macrophages, and neutrophils in the alveolar septa and alveolar lumen, and mild peribronchitis in the lungs. Findings were consistent with clinical infection in the ferrets.
What Should I do With My Pet if I Get Sick?
Although not routinely recommended, testing is available through the USDA National Veterinary Services Laboratory. The CDC recommends keeping pets separated from infected humans and 6 feet away from others to whom virus might be transmitted (CDC, 2020). There is no recommendation to remove pets from homes where SARS-CoV-2 has been detected. In Hong Kong, the Agriculture, Fisheries and Conservation Department recommends quarantining pets if their owners were hospitalized with COVID-19. As of April 15th, only 3 animals (1 cat and 2 dogs) tested positive with 30 dogs, 17 cats and 2 hamsters being quarantined. None of the animals developed respiratory symptoms (AVMA 2020).
Can Pets Get Their Owners Sick?
There do not appear to be any reported cases of human SARS-CoV-2 infection by pets, but further studies are needed to definitively rule out this possibility. There is an example of a single human contracting avian influenza from cats in a NYC animal shelter (Atanaska Marinova-Petkova et al., 2017), which suggests it is possible for a virus, although not specifically SARS-CoV-2 to be transmitted from pets to humans.
Conclusion
We conclude tonight with a celebration of the FLARE team’s pets. These pets can apparently be infected with SARS-CoV-2, but are unlikely to become severely ill and require mechanical ventilation, and there is little evidence to suggest they would become a source of infection for others.
References:
- American Veterinary Medicine Association. “SARS-CoV-2 in animals.” https://www.avma.org/resources-tools/animal-health-and-welfare/covid-19/sars-cov-2-animals-including-pets. 2020.
- Atanaska Marinova-Petkova, Jen Laplante, Yunho Jang, Brian Lynch, Natosha Zanders, Marisela Rodriguez, Joyce Jones, Sharmi Thor, Erin Hodges, Juan A. De La Cruz, et al. (2017). Avian Influenza A(H7N2) Virus in Human Exposed to Sick Cats, New York, USA, 2016. Emerging Infectious Disease Journal 23, 2046.
- Belser, J.A., Eckert, A.M., Tumpey, T.M., and Maines, T.R. (2016). Complexities in Ferret Influenza Virus Pathogenesis and Transmission Models. Microbiol. Mol. Biol. Rev. 80, 733–744.
- CDC (2020). Coronavirus Disease 2019 (COVID-19).
- Halfmann, P.J., Hatta, M., Chiba, S., Maemura, T., Fan, S., Takeda, M., Kinoshita, N., Hattori, S.-I., Sakai-Tagawa, Y., Iwatsuki-Horimoto, K., et al. (2020). Transmission of SARS-CoV-2 in Domestic Cats. N. Engl. J. Med.
- Roberts, A., Vogel, L., Guarner, J., Hayes, N., Murphy, B., Zaki, S., and Subbarao, K. (2005). Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters. J. Virol. 79, 503–511.
- Shi, J., Wen, Z., Zhong, G., Yang, H., Wang, C., Huang, B., Liu, R., He, X., Shuai, L., Sun, Z., et al. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science.
- Sia, S.F., Yan, L.-M., Chin, A.W.H., Fung, K., Choy, K.-T., Wong, A.Y.L., Kaewpreedee, P., Perera, R.A.P.M., Poon, L.L.M., Nicholls, J.M., et al. (2020). Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature.
- Sit, T.H.C., Brackman, C.J., Ip, S.M., Tam, K.W.S., Law, P.Y.T., To, E.M.W., Yu, V.Y.T., Sims, L.D., Tsang, D.N.C., Chu, D.K.W., et al. (2020). Infection of dogs with SARS-CoV-2. Nature.
- Zhang, Q., Zhang, H., Huang, K., Yang, Y., Hui, X., Gao, J., He, X., Li, C., Gong, W., Zhang, Y., et al. (2020). SARS-CoV-2 neutralizing serum antibodies in cats: a serological investigation.
Learn more about research in the Division of Pulmonary and Critical Care Medicine
View all COVID-19 Updates