Pediatric Multisystem Inflammatory Syndrome and COVID-19
The FLARE Four
- Thus far, critical illness due to COVID-19 is rare among pediatric patients. In most case series, pediatric patients represent 1-2% of all COVID-19 cases, with lower illness severity and mortality when compared to adults
- In recent weeks, reports have surfaced of children presenting with a critical illness similar to Kawasaki Shock Syndrome (KDSS) associated with COVID-19 infection. This has been labeled Pediatric Multi-system Inflammatory Syndrome (PMIS) or Multi-system Inflammatory Syndrome in Children (MIS-C)
- Affected children present with a wide spectrum of illness, with the sickest developing shock and respiratory failure and small numbers requiring VA-ECMO. Elevated inflammatory markers (D-dimer, fibrinogen, IL-1, and IL-6) are common. While most patients improve, a few fatalities have been reported
- Treatment has generally been modelled on successful approaches to Kawasaki Disease and include IVIG, aspirin, and consideration of steroids or other immunomodulatory medications. However, no standard has been established
Many people are saying...some children exposed to SARS-CoV-2 have a pathologic inflammatory response.
Subscribe to the latest updates from FLARE Advances in Motion
Introduction
Initial reports from China indicated that COVID-19 largely spared pediatric patients (Liu et al. 2020; Dong et al. 2020), while early reports from the US and Europe indicated that < 5% of symptomatic cases were in children and the number of deaths was vanishingly small (CDC MMWR 2020; Docherty et al. 2020). Similar to their adult counterparts, children with comorbidities (respiratory and cardiovascular diseases, immunosuppression, and obesity) tend to be overrepresented in the symptomatic cohort (Shekerdemian et al. 2020). Recently, clusters of a Kawasaki Shock Syndrome-like illness have been recognized internationally and in many US states where COVID-19 is circulating. Affected children present with a Kawasaki Disease (KD)-like inflammatory syndrome and often progress to shock. Most recover with appropriate critical care interventions, though a few deaths have been reported. This syndrome has been labeled Pediatric Multisystem Inflammatory Syndrome (PMIS) or Multisystem Inflammatory Syndrome (MIS-C).
How Common is COVID-19 in Children?
Rare, though the actual incidence of SARS-CoV-2 infection in pediatric populations is essentially unknown. Pediatric patients account for only 1-2% of COVID-19 cases, but limited testing means these reports may underestimate true incidence. Case series of infection in children have mostly described mild to moderate illness with lower burden of respiratory compromise (reviewed in April 7 FLARE). Even among those pediatric patients admitted to critical care units, the mortality associated with COVID-19 is < 5% (Shekerdemian et al. 2020).
Kawasaki Disease and Kawasaki Shock Syndrome
Kawasaki Disease (KD) is an acute pediatric vasculitis of unknown etiology involving medium-sized vessels (Burns et al. 2000). First described in Japan 50 years ago, it is thought to be a delayed inflammatory syndrome likely resulting from an infectious trigger in a genetically susceptible host. While no specific infectious trigger has been definitively mechanistically linked to KD, there have been prior reports of a Kawasaki disease in the setting of other viral infections, including a previously-identified novel coronavirus (Esper et al. 2005), H1N1 influenza (Joshi et al. 2011), and other respiratory illnesses (Turnier et al. 2015). However, it has historically been difficult to distinguish between coincident, but unrelated, infection and a truly causal relationship between respiratory viral illnesses and Kawasaki Disease. Notably, children of parents who experienced KD are at elevated risk of KD themselves (Uehara et al. 2003), as are those harboring certain variants in immune-related genes (including HLA loci). Together, these findings suggest susceptibility to KD represents a complex interaction between genes and environment.
Classic clinical diagnostic criteria include a persistent fever (> 5 days) and 4 of 5 clinical symptoms including mucocutaneous involvement, non-purulent conjunctivitis, polymorphous rash, unilateral lymphadenopathy and palmar/plantar erythema and desquamation (Sundel 2015). An incomplete form of KD is defined by 3 of the above symptoms as well as a number of inflammatory laboratory data (McCrindle et al. 2017). KD typically affects children < 5 years of age with a predilection for those of Asian descent.
While generally self-limited, KD can have a number of long-term sequelae, the most important of which are cardiovascular. In addition to ventricular and valvular dysfunction, patients with KD can develop coronary aneurysms, which occur in 20-25% of untreated children (McCrindle et al. 2007). Treatment of KD is primarily designed to decrease the likelihood of coronary aneurysm development. In rare cases (5-10%), KD can be accompanied by persistent hypotension during the acute illness in which case it is termed KD Shock Syndrome (KDSS) (Kanegaye et al. 2009).
Reports of PMIS Across the World
Beginning in April 2020, a number of reports emerged describing an increased incidence of KD and KDSS-like illness in pediatric patients. Although the first report involved a 6-month-old child with COVID-19 and a relatively benign course (Jones et al. 2020), the majority of subsequent reports describe older pediatric patients (median age ~ 9-10 years old), frequently with severe cardiovascular involvement. Notably this is older than the typical KD cohort. While some patients have coronary artery aneurysms, many patients also have elevated biomarkers of cardiomyocyte injury and shock physiology - neither typical of KD. Cases have been described in Italy, England, France, Spain and a number of US states, though no systematic US case series has yet been published. Massachusetts has at least 9 recognized cases thus far.
In Italy, the city of Bergamo was the epicenter of the nation’s COVID-19 epidemic. Between February and March 2020, a total of 10 patients fitting the case definition were evaluated at the General Pediatric Unit of Hospital Papa Giovanni XXIII (Verdoni et al. 2020). This incidence is approximately 30-fold higher than had been reported at the center over the prior 5 years. As compared to historical KD cohorts, patients were older (7.5 ± 3.5 vs. 3.0 ± 2.5 years) and a higher fraction had cardiac involvement as evidenced by abnormal echocardiogram (60% vs. 10%) and/or abnormal laboratory studies (80% with elevations in troponin, BNP or both). Notably, two children had positive nasopharyngeal PCR tests for SARS-CoV-2 and 8 children had positive SARS-CoV-2 IgG serologies, of whom three also had detectable IgM. No other viral illness was detected in any of the 10 KDSS-like cases.
A case series from France and Switzerland describes a group of 35 pediatric patients with PMIS cared for at 13 regional hospitals heavily affected by the COVID-19 pandemic (Belhadjer et al. 2020). Patients presented for care between March 22nd and April 30th, 2020. Median age was 10 years (range 2-16 years). Comorbidities were noted in 28% of patients, including obesity in 17%, though none had known cardiovascular disease. Inclusion criteria included fever > 38.5°C, left ventricular dysfunction (EF < 50%) and elevated inflammatory markers. Asthenia was common, and 80% of subjects had gastrointestinal complaints. While all patients had symptoms reminiscent of KD, none met the classic diagnostic criteria. SARS-CoV-2 serologies were positive in 30/35 patients (the majority of which were IgG with only two positive IgM), and 12 had positive nasopharyngeal PCR testing. Notably, these investigators report a median time of 6 days between symptom onset and development of heart failure; 29/35 patients were directly admitted to the ICU, 80% in cardiogenic shock. Management included mechanical ventilation in 62% and V-A ECMO in 28% of patients. Ventricular ejection fraction was <30% in 28% of patients. Coronary dilation was seen in 17% but no aneurysms were noted at the time of publication. All patients received IVIG and a third were treated with steroids. Three patients were given the IL-1 antagonist Anakinra. Median time to resolution of ventricular dysfunction was 2 days. All patients had been successfully weaned from ECMO support and none had died at the time of publication. The authors also note that NT-proBNP was universally elevated and have since established a protocol to evaluate this biomarker in pediatric patients with unexplained persistent fever - thereby identifying a number of milder cases.
A recent case series reports on 8 children aged 4 to 14 years who presented to one hospital in London during a 10 day period in mid-April with “hyperinflammatory shock” (Riphagen et al. 2020). Six were reported to be of African descent, one of East Asian descent, and one of Middle Eastern descent. Fever was noted in all 8 patients, 7 had diarrhea and abdominal complaints, and 7 were found to have myocardial dysfunction. All 8 children tested negative for SARS-CoV-2 on BAL and nasopharyngeal aspirates, but four had direct familial exposures to infected individuals and one tested positive for the virus on post-mortem analysis. Serologic testing for SARS-CoV-2 antibodies is not reported. Adenovirus and enterovirus were isolated in one child, but no other infectious culprits were identified. All 8 had elevated markers of inflammation including CRP, procalcitonin, ferritin, and D-dimer. Four required mechanical ventilation, 3 required non-invasive ventilatory support, and one required VA-ECMO. Five were managed with steroids, one received infliximab, and all 8 received IVIG. One had passed away at the time of publication.
Reports of PMIS in the US have been mostly captured in the lay press. A peer-reviewed case series describing 46 pediatric patients with COVID-19 across 14 American PICUs cared for between March 14th and April 3rd likely preceded the emergence or recognition of this new syndrome (Shekerdemian et al. 2020). Notably, the prevalence of pre-existing conditions in that cohort was 83% and the majority of patients presented in respiratory distress. Mortality among this group of patients was reported at 4.2%. Reports have started to emerge of pediatric patients with PMIS presenting to U.S. hospitals. The CDC has reported greater than 100 cases of PMIS in New York City alone, including 5 deaths. Case definitions for these patients match those described across Europe with high, persistent fever, signs and symptoms of inflammation, frequent development of shock, and laboratory evidence of inflammation and myocardial injury. In our own experience, Boston Children’s Hospital has to date cared for nearly 20 patients with PMIS. Of these, 2 developed severe, and 4 others mild, ventricular dysfunction. One patient had a transient complete heart block. All patients have had normalization of ventricular function. No patients thus far at Boston Children’s have required ECMO or died.
What is the Case Definition of PMIS?
Given the novel nature of the illness and multiple small case-series emerging about PMIS, the case definition can vary slightly. A schematic is shown below (Figure 1). In general, clinical characteristics include the following:
Pediatric patient (WHO: 0-19 years, CDC: <21 years of age)
AND
Fever (WHO: 3 or more days. CDC: “persistent”)
AND two of the following:
- Rash or bilateral non-purulent conjunctivitis or muco-cutaneous inflammation signs (oral, hands or feet)
- Hypotension or shock
- Features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities (including ECHO findings or elevated Troponin/NT-proBNP)
- Evidence of coagulopathy (by PT, PTT, elevated D-dimer)
- Acute gastrointestinal problems (diarrhea, vomiting, or abdominal pain)
AND
Elevated markers of inflammation such as ESR, C-reactive protein, or procalcitonin
AND
No other obvious microbial cause of inflammation, including bacterial sepsis, staphylococcal or streptococcal shock syndromes
AND
Evidence of COVID-19 (RT-PCR, antigen test or serology positive), or likely contact with patient with COVID-19
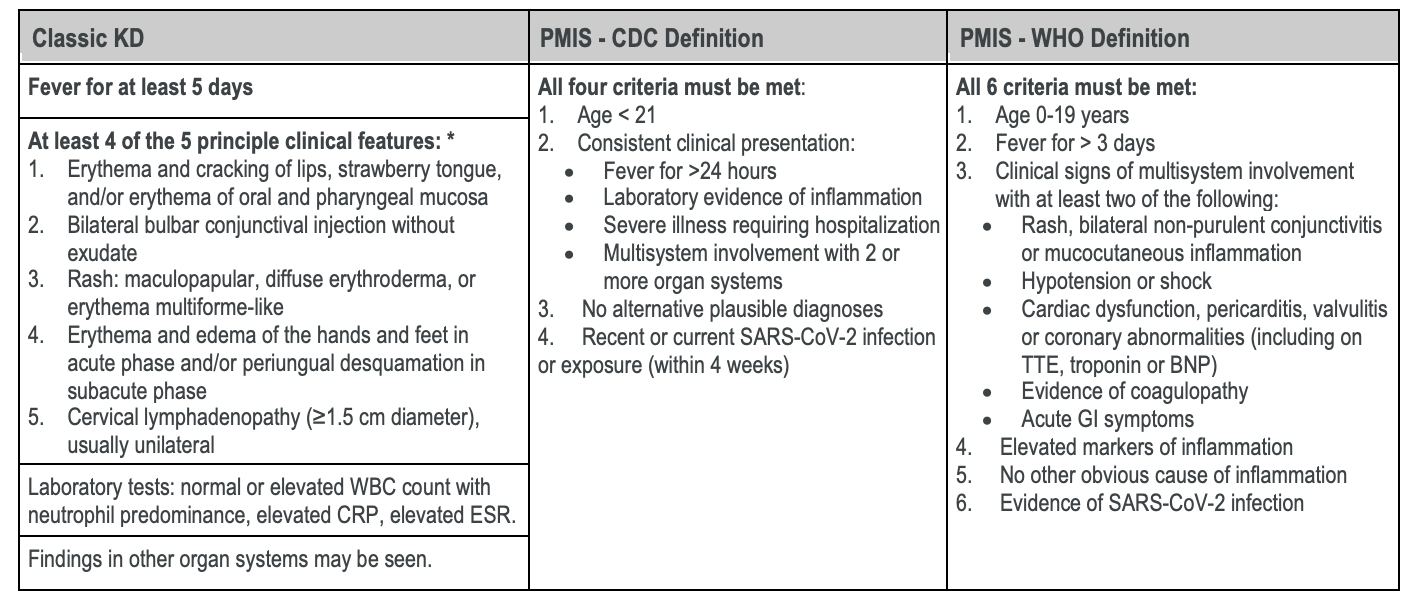
Table 1
Comparison of features used in the diagnosis of Classic KD, incomplete KD and the case definitions of PMIS. Adapted from (McCrindle et al. 2017; CDC Case Definition, WHO Case Definition).
*Incomplete KD is characterized by fewer than four signs of mucocutaneous inflammation; may also include infants with fevers for ≥ 7 days without alternative explanation.
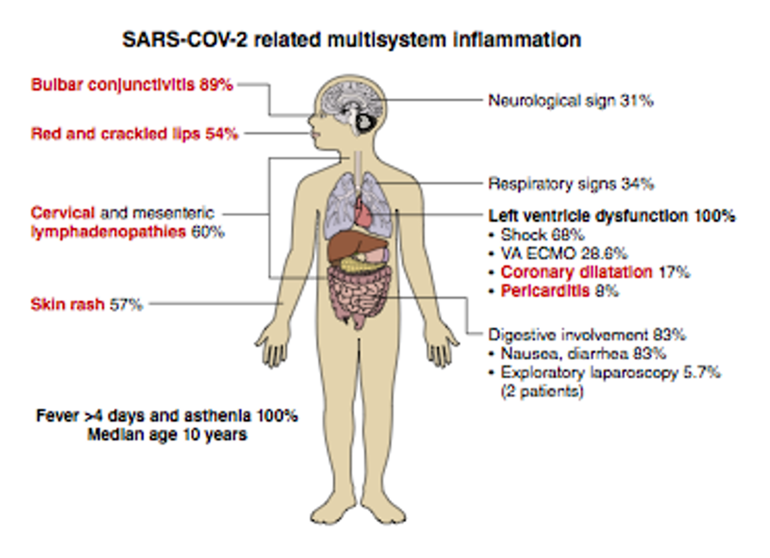
Figure 1
Schematic of characteristics of PMIS. From Belhadjer et al. 2020.
Is PMIS the Same as COVID-19, the Same as Kawasaki Disease, or a New Syndrome?
As discussed above, the clinical syndrome of PIMS/MIS-C resembles many features of Kawasaki Disease. Infections are common at the time of diagnosis of Kawasaki Disease (Benseler et al. 2005) and it is thought that the disease starts with an innate immune response to a particular antigen and then progress to a hyperinflammatory state characterized by a unique combination of IgA secreting plasma cells and macrophages. No single risk factor for Kawasaki Disease explains more than a small percentage of the overall risk of contracting the disease (Sundel 2015). Since some aspects of the clinical presentation of PMIS/MIS-C resemble those of Kawasaki Disease, it has been proposed that the pathophysiology is similar. Evidence in support of the hypothesis that PMIS/MIS-C is in some way a post-viral autoimmune process comes from serologic studies. However, it remains unclear whether PMIS/MIS-C patients present with an active SARS-CoV-2 infection because most case series PMIS/MISC-C use a definition of COVID-19 that encompasses both positive PCR testing and/or positive serologic testing. In a French series of 35 patients with PIMS/MIS-C, 30/35 patients had positive serologic assays (Belhadjer et al. 2020). Of these, 23 had IgA and IgG, 3 had IgG, 2 had IgG and IgM, and 2 had IgA only. The high prevalence of IgG and low prevalence of IgM does suggest that SARS-CoV-2 infection was not recent in these patients. The delay between the peak of the pandemic in the general population and the emergence of PMIS/MIS-C is also consistent with a hypothesis that PMIS may be a delayed syndrome after recovery from SARS-CoV-2 infection. Moreover, some have even argued that emergence of COVID-19-associated multisystem inflammatory syndrome is not surprising and should simply be called and managed as Kawasaki Disease, albeit caused by a novel virus (Loomba, Villarreal, and Flores 2020).
Treatment
Treatment for PIMS/MIS-C, to date, is largely based on treatment for KD. Intravenous immunoglobulin (dose generally 2g/kg), which has long been first-line therapy for KD (Kobayashi et al. 2012), has been the most commonly used medication in this new, emerging syndrome due to its broad immunomodulatory effects (Lo and Newburger 2018). Other immunomodulatory medications that have been suggested include corticosteroids, tumor necrosis factor-alpha blockers, IL-1 inhibitors, and others. However, there is almost no data to support any particular approach.
Conclusions
The emergence and now widespread incidence of SARS-CoV-2 has led to nearly 5 million cases and more than 300,000 deaths worldwide. With this expansion in disease burden, our understanding of the disease is also rapidly evolving. Although children are affected in relatively small numbers, some may be critically affected by KDSS-like PMIS, with development of rapidly progressive vasoplegic and cardiogenic shock requiring vasopressors, mechanical ventilation, and at times, mechanical circulatory support. While the exact relationship to SARS-CoV-2 remains murky, the majority of affected patients have a known contact with COVID-19 or serologies demonstrating prior infection with SARS-CoV-2; a minority appear to be actively infected. As in Kawasaki Disease, this illness may represent a post-infectious inflammatory syndrome with associated cardiac dysfunction and coronary aneurysms. Although data to support specific therapies are lacking, proposed treatment follows KD treatment protocols and aims to decrease inflammation. The emergence of this severe syndrome has prompted the organization of multinational expert groups to improve understanding and management of affected patients with standardized and prospectively evaluated protocols. In Massachusetts, the Department of Public Health has deemed PMIS a mandatory reportable illness as of May 14th. Fortunately, with appropriate treatment, the majority of patients appear to make significant recovery, though long-term sequelae are yet to be determined.
References:
- Belhadjer, Zahra, Mathilde Méot, Fanny Bajolle, Diala Khraiche, Antoine Legendre, Samya Abakka, Johanne Auriau, et al. 2020. “Acute Heart Failure in Multisystem Inflammatory Syndrome in Children (MIS-C) in the Context of Global SARS-CoV-2 Pandemic.” Circulation, May. https://doi.org/10.1161/CIRCULATIONAHA.120.048360.
- Benseler, Susanne M., Brian W. McCrindle, Earl D. Silverman, Pascal N. Tyrrell, Joseph Wong, and Rae S. M. Yeung. 2005. “Infections and Kawasaki Disease: Implications for Coronary Artery Outcome.” Pediatrics 116 (6): e760–66.
- Burns, J. C., H. I. Kushner, J. F. Bastian, H. Shike, C. Shimizu, T. Matsubara, and C. L. Turner. 2000. “Kawasaki Disease: A Brief History.” Pediatrics 106 (2): E27.
- CDC MMWR. 2020. “Coronavirus Disease 2019 in Children — United States, February 12–April 2, 2020.” MMWR. Morbidity and Mortality Weekly Report 69. https://doi.org/10.15585/mmwr.mm6914e4.
- Docherty, Annemarie B., Ewen M. Harrison, Christopher A. Green, Hayley E. Hardwick, Riinu Pius, Lisa Norman, Karl A. Holden, et al. 2020. “Features of 16,749 Hospitalised UK Patients with COVID-19 Using the ISARIC WHO Clinical Characterisation Protocol.” medRxiv, April, 2020.04.23.20076042.
- Dong, Yuanyuan, Xi Mo, Yabin Hu, Xin Qi, Fang Jiang, Zhongyi Jiang, and Shilu Tong. 2020. “Epidemiological Characteristics of 2143 Pediatric Patients With 2019 Coronavirus Disease in China.” Pediatrics, March. https://doi.org/10.1542/peds.2020-0702.
- Esper, Frank, Eugene D. Shapiro, Carla Weibel, David Ferguson, Marie L. Landry, and Jeffrey S. Kahn. 2005. “Association between a Novel Human Coronavirus and Kawasaki Disease.” The Journal of Infectious Diseases 191 (4): 499–502.
- Jones, Veena G., Marcos Mills, Dominique Suarez, Catherine A. Hogan, Debra Yeh, J. Bradley Segal, Elizabeth L. Nguyen, Gabrielle R. Barsh, Shiraz Maskatia, and Roshni Mathew. 2020. “COVID-19 and Kawasaki Disease: Novel Virus and Novel Case.” Hospital Pediatrics, April. https://doi.org/10.1542/hpeds.2020-0123.
- Joshi, Archana V., Kelsey D. J. Jones, Ann-Marie Buckley, Michael E. Coren, and Beate Kampmann. 2011. “Kawasaki Disease Coincident with Influenza A H1N1/09 Infection: Kawasaki Disease with H1N1/09 Infection.” Pediatrics International: Official Journal of the Japan Pediatric Society 53 (1): e1–2.
- Kanegaye, John T., Matthew S. Wilder, Delaram Molkara, Jeffrey R. Frazer, Joan Pancheri, Adriana H. Tremoulet, Virginia E. Watson, Brookie M. Best, and Jane C. Burns. 2009. “Recognition of a Kawasaki Disease Shock Syndrome.” Pediatrics 123 (5): e783–89.
- Kobayashi, Tohru, Tsutomu Saji, Tetsuya Otani, Kazuo Takeuchi, Tetsuya Nakamura, Hirokazu Arakawa, Taichi Kato, et al. 2012. “Efficacy of Immunoglobulin plus Prednisolone for Prevention of Coronary Artery Abnormalities in Severe Kawasaki Disease (RAISE Study): A Randomised, Open-Label, Blinded-Endpoints Trial.” The Lancet 379 (9826): 1613–20.
- Liu, Weiyong, Qi Zhang, Junbo Chen, Rong Xiang, Huijuan Song, Sainan Shu, Ling Chen, et al. 2020. “Detection of Covid-19 in Children in Early January 2020 in Wuhan, China.” The New England Journal of Medicine 382 (14): 1370–71.
- Lo, Mindy S., and Jane W. Newburger. 2018. “Role of Intravenous Immunoglobulin in the Treatment of Kawasaki Disease.” International Journal of Rheumatic Diseases 21 (1): 64–69.
- Loomba, Rohit S., Enrique Villarreal, and Saul Flores. 2020. “Covid-19 and Kawasaki Syndrome: Should We Really Be Surprised?” Cardiology in the Young, May, 1–5.
- McCrindle, Brian W., Jennifer S. Li, L. Luann Minich, Steven D. Colan, Andrew M. Atz, Masato Takahashi, Victoria L. Vetter, et al. 2007. “Coronary Artery Involvement in Children with Kawasaki Disease: Risk Factors from Analysis of Serial Normalized Measurements.” Circulation 116 (2): 174–79.
- McCrindle, Brian W., Anne H. Rowley, Jane W. Newburger, Jane C. Burns, Anne F. Bolger, Michael Gewitz, Annette L. Baker, et al. 2017. “Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association.” Circulation 135 (17): e927–99.
- “Multisystem Inflammatory Syndrome in Children and Adolescents with COVID-19.” n.d. Accessed May 21, 2020. https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19.
- Riphagen, Shelley, Xabier Gomez, Carmen Gonzalez-Martinez, Nick Wilkinson, and Paraskevi Theocharis. 2020. “Hyperinflammatory Shock in Children during COVID-19 Pandemic.” The Lancet, May. https://doi.org/10.1016/S0140-6736(20)31094-1.
- Shekerdemian, Lara S., Nabihah R. Mahmood, Katie K. Wolfe, Becky J. Riggs, Catherine E. Ross, Christine A. McKiernan, Sabrina M. Heidemann, et al. 2020. “Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units.” JAMA Pediatrics, May. https://doi.org/10.1001/jamapediatrics.2020.1948.
- Sundel, Robert P. 2015. “Kawasaki Disease.” Rheumatic Diseases Clinics of North America 41 (1): 63–73, viii.
- Turnier, Jessica L., Marsha S. Anderson, Heather R. Heizer, Pei-Ni Jone, Mary P. Glodé, and Samuel R. Dominguez. 2015. “Concurrent Respiratory Viruses and Kawasaki Disease.” Pediatrics 136 (3): e609–14.
- Uehara, R., M. Yashiro, Y. Nakamura, and H. Yanagawa. 2003. “Kawasaki Disease in Parents and Children.” Acta Paediatrica 92 (6): 694–97.
- Verdoni, Lucio, Angelo Mazza, Annalisa Gervasoni, Laura Martelli, Maurizio Ruggeri, Matteo Ciuffreda, Ezio Bonanomi, and Lorenzo D’Antiga. 2020. “An Outbreak of Severe Kawasaki-like Disease at the Italian Epicentre of the SARS-CoV-2 Epidemic: An Observational Cohort Study.” The Lancet, May. https://doi.org/10.1016/S0140-6736(20)31103-X.
Learn more about research in the Division of Pulmonary and Critical Care Medicine
View all COVID-19 Updates