FLARE: The Interplay Between Diabetes and SARS-CoV-2
The FLARE Four
- Observational data indicate that patients with diabetes are at higher risk for severe COVID-19 illness and death
- It is possible that an interaction between the inflammatory response to SARS-CoV-2 and acute hyperglycemia contributes to the morbidity and mortality seen in patients with diabetes and COVID-19
- Efforts to improve blood glucose monitoring and management in critically ill patients may therefore improve outcomes in COVID-19
- More research is needed to determine if there are factors intrinsic to SARS-CoV-2 that interact with diabetes and lead to more severe presentations
Many people are saying...diabetes makes COVID-19 worse
Subscribe to the latest updates from Advances in Motion
Introduction
Case series detailing risk factors for severe COVID-19 consistently identify diabetes mellitus (Grasselli et al. 2020; Goyal et al. 2020). The CDC has advised that patients with diabetes are at higher risk for severe COVID-19 illness. Does having diabetes make COVID-19 presentations more severe? Relatedly, does having COVID-19 make diabetes presentations more severe?
Does diabetes make COVID-19 presentations more severe?
During past viral epidemics, including those caused by SARS-CoV-1, H1N1 influenza, and MERS-CoV, diabetes has been associated with increased mortality and morbidity (J. K. Yang et al. 2006; Kulcsar et al. 2019; Allard et al. 2010). Initial studies emerging from China have reported high prevalence of comorbidities including diabetes among patients admitted with COVID-19. The table below compares the rates of diabetes in the general population across different countries to rates of diabetes in large case series of patients with COVID-19. Briefly, across all studies, the prevalence of diabetes in COVID-19 is generally higher than that in the general population. Of note, most studies to date have not differentiated between type 1 diabetes and type 2 diabetes.
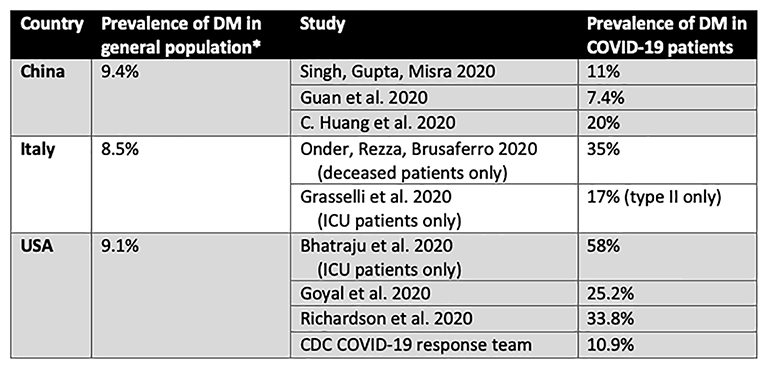
Table
Comparison of rates of diabetes in general population to those reported in large case series of COVID-19 patients in different countries. *(“WHO | Diabetes Country Profiles 2016”, 2016)
Furthermore, patients with diabetes appear to be at higher risk not only for contracting SARS-CoV-2, but also for developing severe COVID-19 and succumbing to the illness. As illustrated in Figures 1 and 2 below, across several studies reviewed by Singh et al., patients with diabetes demonstrated substantially higher rates of severe COVID-19 and death (Singh, Gupta, and Misra 2020).
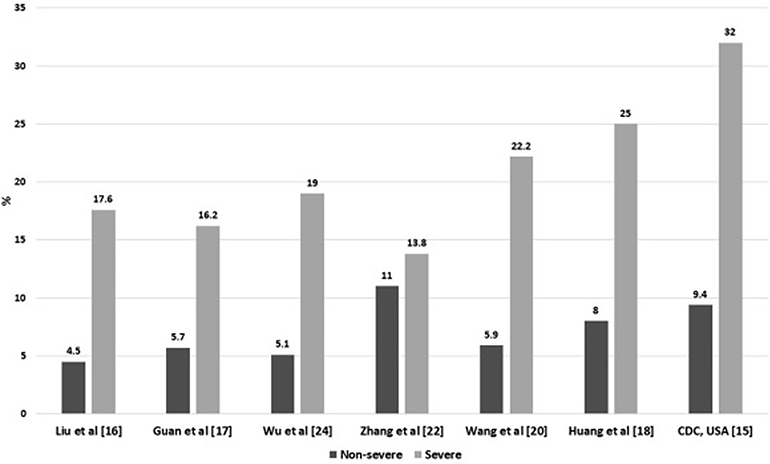
Figure 1
Rates of severe vs. non-severe COVID-19 infection in diabetics (Singh, Gupta, and Misra 2020).
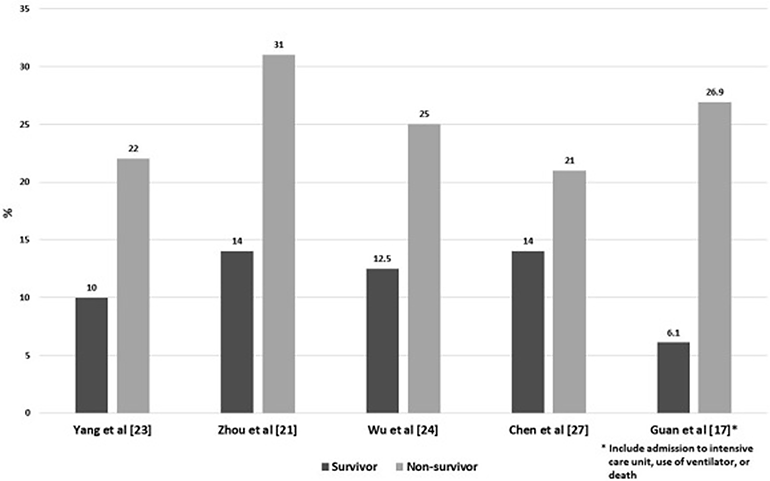
Figure 2
Prevalence of diabetes in survivors versus non-survivors (Singh, Gupta, and Misra 2020).
Many additional reports have suggested an association between diabetes and severe COVID-19. Wu et al., in an analysis of 201 patients, found that diabetes was associated with development of acute respiratory distress syndrome (ARDS), with a hazard ratio of 2.34 (Wu et al. 2020). In a systematic review of 13 studies, 3,027 patients with COVID-19 were analyzed to determine risk factors for critical or lethal illness, revealing a 3.7-fold increased odds of diabetes in the critically ill (Zheng et al. 2020). In an even larger systematic review, nearly 6,500 patients (representing 30 studies) were analyzed to identify statistically significant associations between diabetes and severe COVID-19 (RR 2.5), ARDS (RR 4.6), death (RR 2.1), and composite poor outcome (RR 2.4), though the authors note the possibility of confounding by age and hypertension (I. Huang, Lim, and Pranata 2020).
In an effort to eliminate potential confounders from the analysis, an observational study by Gou et al. divided patients with COVID-19 into groups of those without diabetes (n=26) and those with only diabetes and no other comorbidities (n=24). However, the median age of patients with diabetes was 61, compared to the median age of 32 for patients without diabetes, making the comparison very difficult (Guo et al. 2020). Thus, the increased prevalence of patients with diabetes in COVID-19 cohorts has revealed an association between these two diseases, but no causal link has been established. There are many possible confounders including differences in age and socioeconomic status, as well as concurrent comorbidities such as hypertension and obesity.
How might diabetes exacerbate COVID-19?
Although causality has yet to be definitively established, several mechanisms have been proposed to explain how diabetes and hyperglycemia might lead to more severe viral respiratory illnesses in particular. Hyperglycemia can reduce neutrophil degranulation, impair complement activation, and impair phagocytosis, all of which can increase severity of viral infections. Elevated blood glucose levels can also directly increase glucose concentrations in airway secretions (Philips et al. 2003) and, based on in vitro studies of influenza, may thereby promote viral replication (Kohio and Adamson 2013). Other studies have suggested that hyperglycemia can damage cells, increase concentrations of toxic intracellular by-products of the glycolytic pathway, and ultimately contribute to the cytokine and inflammatory response, worsening prognosis of acute viral infections (Gentile, Strollo, and Ceriello 2020). As a result, during the current COVID-19 pandemic, many have advocated for better monitoring and management of blood sugar in patients with COVID-19 (Klonoff and Umpierrez 2020; Ma and Ran 2020; Zhou and Tan 2020).
Another hypothesis is that diabetes might regulate COVID-19 severity by modulating the expression of angiotensin-converting enzyme 2 (ACE2), a cell-surface receptor for SARS-CoV-2 (Hoffmann et al. 2020; Lu et al. 2020). Interestingly, acute hyperglycemia has been shown to upregulate ACE2 expression in the kidneys of leptin receptor-deficient mice (Wysocki et al. 2006) and the serum, liver, and pancreas of non-obese diabetic mice (Roca-Ho et al. 2017). Some anti-diabetic drugs, including liraglutide and pioglitazone, have also been associated with ACE2 upregulation in animal studies (Muniyappa and Gubbi 2020; Romaní-Pérez et al. 2015). It remains unknown whether ACE2 levels in humans are likewise regulated or whether such regulation would alter susceptibility to SARS-CoV-2 infection.
Does COVID-19 worsen diabetes control?
Based on the observational data detailed above, patients with diabetes do appear to be at an increased risk for severe COVID-19. What is less clear is whether having COVID-19 makes manifestations of diabetes more severe, such as increased presentations of diabetic ketoacidosis (DKA) or significant increase of insulin resistance. In a recent review in Lancet Diabetes and Endocrinology, the authors shared their observation of high insulin requirements in patients with severe COVID-19, and their personal view that the extent of insulin resistance in patients with diabetes seems disproportionate compared with critical illness caused by other conditions (Bornstein et al. 2020). However, these are simply the impressions of the authors, not primary data. Li et al. recently reported that COVID-19 infection may cause ketosis and ketoacidosis. Out of 658 hospitalized patients with confirmed COVID-19, 42 presented with ketosis. 15 of the 42 patients with ketosis had diabetes (only one with type 1 diabetes) and 3 out of the 15 cases developed diabetic ketoacidosis (DKA). They concluded that COVID-19 may induce DKA in patients with type 2 diabetes (Li et al. 2020). Unfortunately, the sample size of this study was small and there was no proposed mechanism as to how COVID-19 may predispose to DKA.
Acute infection is a known trigger for DKA, typically in the setting of concurrent dehydration and insensible losses. The acute cytokine release and inflammatory reaction triggered by infection is also known to contribute to insulin resistance and beta-cell dysfunction (Hasnain et al. 2014; Frydrych et al. 2017). COVID-19 may predispose patients to severe hyperglycemia and DKA like any other acute infection, or COVID-19 may uniquely increase this risk, particularly in type 2 diabetes.
There is a hypothesis that coronaviruses cause transient beta-cell dysfunction, leading to acute hyperglycemia and relative insulin deficiency, predisposing to DKA. In a study on SARS coronavirus (SARS-CoV-1) by Yang et al. detected ACE2 expression in the endocrine pancreas. Clinically, thirty-nine patients with SARS who had no history of type 2 diabetes and who received no glucocorticoid therapy were compared to their healthy siblings during a 3-year follow up period. More than 50% of the patients (20 out of 39) developed diabetes (diagnosed by fasting glucose) within 2 weeks of their hospitalization for SARS, but only two (5%) remained diabetic after three years of recovery. Given the expression of ACE2 in the pancreas (Figure 3), they proposed that binding of SARS-CoV-1 to ACE2 allows for entry into islet cells and subsequent damage causing acute diabetes (J.-K. Yang et al. 2010). Based on what we know about the similarity of binding proteins and cellular entry between SARS-CoV-1 and SARS-CoV-2 (Hoffmann et al. 2020; Lu et al. 2020), SARS-CoV-2 may have a similar effect on pancreatic islet cells in patients with COVID-19. In patients with known diabetes, the effects of beta-cell dysregulation may lead to acutely worsening hyperglycemia, higher insulin needs, and ketoacidosis, while those without known diabetes may present with new-onset disease. This hypothesis will be thoroughly tested as we continue to learn about the presence of SARS-CoV-2 in different tissues from pathology reports.
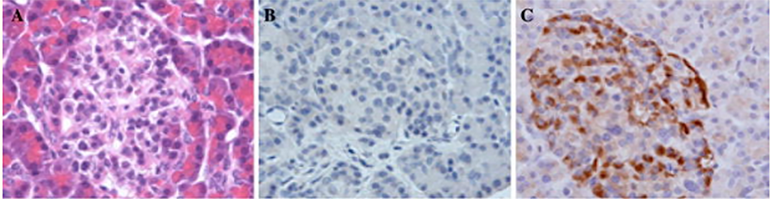
Figure 3: Staining of the human pancreas for ACE2 protein.
(a) H&E staining of pancreatic exocrine tissue (red) with a pancreatic islet (in the middle). (b) Negative immunostaining control. (c) IHC staining of pancreatic tissue is strongly positive for ACE2 (J.-K. Yang et al. 2010).
The proposed relationship between ACE2 and SARS-CoV-2 suggests the possible existence of a feedback loop in which: (1) SARS-CoV-2 virus infects the lung and pancreas via ACE2; (2) infection leads to severe COVID-19 illness and severe hyperglycemia, respectively; (3) severe hyperglycemia may, in turn, lead to upregulation of ACE2 in various organs, (4) upregulation of ACE2 leads to further viral entry and further inflammation (Brufsky 2020).
Conclusions
Observational data suggests that patients with diabetes develop severe COVID-19 more often than patients without diabetes. Whether SARS-CoV-2 has intrinsic properties that affect the pathophysiology of glycemic regulation in hosts and makes diabetes more severe in patients with COVID-19 is yet to be determined. It is possible that a vicious cycle develops, where the virulence properties of SARS-CoV-2 and acutely impaired glycemic control in patients with diabetes directly contribute to the morbidity and mortality of patients with COVID-19.
Future studies are needed to determine the importance of and parameters for glycemic control in this specific population. It may not be that COVID-19 patients require an alternative management strategy to currently accepted blood glucose management protocols (e.g. NICE-SUGAR Study Investigators et al. 2009), but rather that clinicians should practice with heightened awareness of potential complications from diabetes and SARS-CoV-2.
References
- Allard, Robert, Pascale Leclerc, Claude Tremblay, and Terry-Nan Tannenbaum. 2010. “Diabetes and the Severity of Pandemic Influenza A (H1N1) Infection.” Diabetes Care 33 (7): 1491–93.
- Bhatraju, Pavan K., Bijan J. Ghassemieh, Michelle Nichols, Richard Kim, Keith R. Jerome, Arun K. Nalla, Alexander L. Greninger, et al. 2020. “Covid-19 in Critically Ill Patients in the Seattle Region—case Series.” The New England Journal of Medicine. https://www.nejm.org/doi/full/10.1056/NEJMoa2004500.
- Bornstein, Stefan R., Francesco Rubino, Kamlesh Khunti, Geltrude Mingrone, David Hopkins, Andreas L. Birkenfeld, Bernhard Boehm, et al. 2020. “Practical Recommendations for the Management of Diabetes in Patients with COVID-19.” The Lancet. Diabetes & Endocrinology, April. https://doi.org/10.1016/S2213-8587(20)30152-2.
- Brufsky, Adam. 2020. “Hyperglycemia, Hydroxychloroquine, and the COVID-19 Epidemic.” Journal of Medical Virology. https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.25887.
- Frydrych, Lynn M., Fatemeh Fattahi, Katherine He, Peter A. Ward, and Matthew J. Delano. 2017. “Diabetes and Sepsis: Risk, Recurrence, and Ruination.” Frontiers in Endocrinology 8 (October): 271.
- Gentile, Sandro, Felice Strollo, and Antonio Ceriello. 2020. “COVID-19 Infection in Italian People with Diabetes: Lessons Learned for Our Future (an Experience to Be Used).” Diabetes Research and Clinical Practice.
- Goyal, Parag, Justin J. Choi, Laura C. Pinheiro, Edward J. Schenck, Ruijun Chen, Assem Jabri, Michael J. Satlin, et al. 2020. “Clinical Characteristics of Covid-19 in New York City.” The New England Journal of Medicine, April. https://doi.org/10.1056/NEJMc2010419.
- Grasselli, Giacomo, Alberto Zangrillo, Alberto Zanella, Massimo Antonelli, Luca Cabrini, Antonio Castelli, Danilo Cereda, et al. 2020. “Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy.” JAMA: The Journal of the American Medical Association, April. https://doi.org/10.1001/jama.2020.5394.
- Guan, Wei-Jie, Zheng-Yi Ni, Yu Hu, Wen-Hua Liang, Chun-Quan Ou, Jian-Xing He, Lei Liu, et al. 2020. “Clinical Characteristics of Coronavirus Disease 2019 in China.” The New England Journal of Medicine, February. https://doi.org/10.1056/NEJMoa2002032.
- Guo, Weina, Mingyue Li, Yalan Dong, Haifeng Zhou, Zili Zhang, Chunxia Tian, Renjie Qin, et al. 2020. “Diabetes Is a Risk Factor for the Progression and Prognosis of COVID-19.” Diabetes/metabolism Research and Reviews, March, e3319.
- Hasnain, Sumaira Z., Danielle J. Borg, Brooke E. Harcourt, Hui Tong, Yonghua H. Sheng, Choa Ping Ng, Indrajit Das, et al. 2014. “Glycemic Control in Diabetes Is Restored by Therapeutic Manipulation of Cytokines That Regulate Beta Cell Stress.” Nature Medicine 20 (12): 1417–26.
- Hoffmann, Markus, Hannah Kleine-Weber, Simon Schroeder, Nadine Krüger, Tanja Herrler, Sandra Erichsen, Tobias S. Schiergens, et al. 2020. “SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor.” Cell, March. https://doi.org/10.1016/j.cell.2020.02.052.
- Huang, Chaolin, Yeming Wang, Xingwang Li, Lili Ren, Jianping Zhao, Yi Hu, Li Zhang, et al. 2020. “Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China.” The Lancet 395 (10223): 497–506.
- Huang, Ian, Michael Anthonius Lim, and Raymond Pranata. 2020. “Diabetes Mellitus Is Associated with Increased Mortality and Severity of Disease in COVID-19 Pneumonia - A Systematic Review, Meta-Analysis, and Meta-Regression.” Diabetes & Metabolic Syndrome 14 (4): 395–403.
- Klonoff, David C., and Guillermo E. Umpierrez. 2020. “COVID-19 in Patients with Diabetes: Risk Factors That Increase Morbidity.” Metabolism: Clinical and Experimental, April, 154224.
- Kohio, Hinissan P., and Amy L. Adamson. 2013. “Glycolytic Control of Vacuolar-Type ATPase Activity: A Mechanism to Regulate Influenza Viral Infection.” Virology 444 (1-2): 301–9.
- Kulcsar, Kirsten A., Christopher M. Coleman, Sarah E. Beck, and Matthew B. Frieman. 2019. “Comorbid Diabetes Results in Immune Dysregulation and Enhanced Disease Severity Following MERS-CoV Infection.” JCI Insight 4 (20). https://doi.org/10.1172/jci.insight.131774.
- Li, Juyi, Xiufang Wang, Jian Chen, Xiuran Zuo, Hongmei Zhang, and Aiping Deng. 2020. “COVID -19 Infection May Cause Ketosis and Ketoacidosis.” Diabetes, Obesity and Metabolism. https://doi.org/10.1111/dom.14057.
- Lu, Roujian, Xiang Zhao, Juan Li, Peihua Niu, Bo Yang, Honglong Wu, Wenling Wang, et al. 2020. “Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding.” The Lancet 395 (10224): 565–74.
- Ma, W. X., and X. W. Ran. 2020. “The Management of Blood Glucose Should Be Emphasized in the Treatment of COVID-19.” Sichuan Da Xue Xue Bao. Yi Xue Ban= Journal of Sichuan University. Medical Science Edition 51 (2): 146–50.
- Muniyappa, Ranganath, and Sriram Gubbi. 2020. “COVID-19 Pandemic, Coronaviruses, and Diabetes Mellitus.” American Journal of Physiology-Endocrinology and Metabolism 318 (5): E736–41.
- NICE-SUGAR Study Investigators, Simon Finfer, Dean R. Chittock, Steve Yu-Shuo Su, Deborah Blair, Denise Foster, Vinay Dhingra, et al. 2009. “Intensive versus Conventional Glucose Control in Critically Ill Patients.” The New England Journal of Medicine 360 (13): 1283–97.
- Onder, Graziano, Giovanni Rezza, and Silvio Brusaferro. 2020. “Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy.” JAMA: The Journal of the American Medical Association, March. https://doi.org/10.1001/jama.2020.4683.
- Philips, Barbara J., Jean-Xavier Meguer, Jonathan Redman, and Emma H. Baker. 2003. “Factors Determining the Appearance of Glucose in Upper and Lower Respiratory Tract Secretions.” Intensive Care Medicine 29 (12): 2204–10.
- Richardson, Safiya, Jamie S. Hirsch, Mangala Narasimhan, James M. Crawford, Thomas McGinn, Karina W. Davidson, Douglas P. Barnaby, et al. 2020. “Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area.” JAMA: The Journal of the American Medical Association, April. https://doi.org/10.1001/jama.2020.6775.
- Roca-Ho, Heleia, Marta Riera, Vanesa Palau, Julio Pascual, and Maria Jose Soler. 2017. “Characterization of ACE and ACE2 Expression within Different Organs of the NOD Mouse.” International Journal of Molecular Sciences 18 (3). https://doi.org/10.3390/ijms18030563.
- Romaní-Pérez, Marina, Verónica Outeiriño-Iglesias, Christian M. Moya, Pilar Santisteban, Lucas C. González-Matías, Eva Vigo, and Federico Mallo. 2015. “Activation of the GLP-1 Receptor by Liraglutide Increases ACE2 Expression, Reversing Right Ventricle Hypertrophy, and Improving the Production of SP-A and SP-B in the Lungs of Type 1 Diabetes Rats.” Endocrinology. https://doi.org/10.1210/en.2014-1685.
- Singh, Awadhesh Kumar, Ritesh Gupta, and Anoop Misra. 2020. “Comorbidities in COVID-19: Outcomes in Hypertensive Cohort and Controversies with Renin Angiotensin System Blockers.” Diabetes & Metabolic Syndrome 14 (4): 283–87.
- Singh, A. K., Gupta, R., Ghosh, A., & Misra, A. (2020). Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr, 14(4), 303-310. doi:10.1016/j.dsx.2020.04.004
- Team, Cdc Covid-19 Response, CDC COVID-19 Response Team, Nancy Chow, Katherine Fleming-Dutra, Ryan Gierke, Aron Hall, Michelle Hughes, et al. 2020. “Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 — United States, February 12–March 28, 2020.” MMWR. Morbidity and Mortality Weekly Report. https://doi.org/10.15585/mmwr.mm6913e2.
- “WHO | Diabetes Country Profiles 2016.” 2016, April. https://www.who.int/diabetes/country-profiles/en/.
- Wu, Chaomin, Xiaoyan Chen, Yanping Cai, Jia ’an Xia, Xing Zhou, Sha Xu, Hanping Huang, et al. 2020. “Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China.” JAMA Internal Medicine, March. https://doi.org/10.1001/jamainternmed.2020.0994.
- Wysocki, Jan, Minghao Ye, Maria José Soler, Susan B. Gurley, Hong D. Xiao, Kenneth E. Bernstein, Thomas M. Coffman, Sheldon Chen, and Daniel Batlle. 2006. “ACE and ACE2 Activity in Diabetic Mice.” Diabetes 55 (7): 2132–39.
- Yang, Jin-Kui, Shan-Shan Lin, Xiu-Juan Ji, and Li-Min Guo. 2010. “Binding of SARS Coronavirus to Its Receptor Damages Islets and Causes Acute Diabetes.” Acta Diabetologica 47 (3): 193–99.
- Yang, J. K., Y. Feng, M. Y. Yuan, S. Y. Yuan, H. J. Fu, B. Y. Wu, G. Z. Sun, et al. 2006. “Plasma Glucose Levels and Diabetes Are Independent Predictors for Mortality and Morbidity in Patients with SARS.” Diabetic Medicine: A Journal of the British Diabetic Association 23 (6): 623–28.
- Zheng, Zhaohai, Fang Peng, Buyun Xu, Jingjing Zhao, Huahua Liu, Jiahao Peng, Qingsong Li, et al. 2020. “Risk Factors of Critical & Mortal COVID-19 Cases: A Systematic Literature Review and Meta-Analysis.” The Journal of Infection, April. https://doi.org/10.1016/j.jinf.2020.04.021.
- Zhou, Jun, and Jie Tan. 2020. “Diabetes Patients with COVID-19 Need Better Blood Glucose Management in Wuhan, China.” Metabolism: Clinical and Experimental 107 (March): 154216.
View all COVID-19 Updates
Sign up for FLARE updates