Fluid Management in ARDS
The FLARE Four
- The three best evidence-based interventions in ARDS are lung-protective ventilation (March 23 FLARE), prone positioning (March 29 FLARE), and conservative fluid management
- Conservative fluid management is generally defined and guided by the results of the landmark 2006 FACTT (Fluid and Catheter Treatment Trial) study and includes avoidance of positive fluid balance and normalization of intravascular volume in patients post-resuscitation and without ongoing shock (ARDS Clinical Trials Network, 2006)
- In addition to conservative management post-resuscitation, it is appropriate to guide fluid resuscitation by measures of volume responsiveness
- Volume responsiveness should be assessed with dynamic rather than static measures
Subscribe to the latest updates from FLARE Advances in Motion
Many people are saying...we should diurese patients with COVID-19 ARDS aggressively.
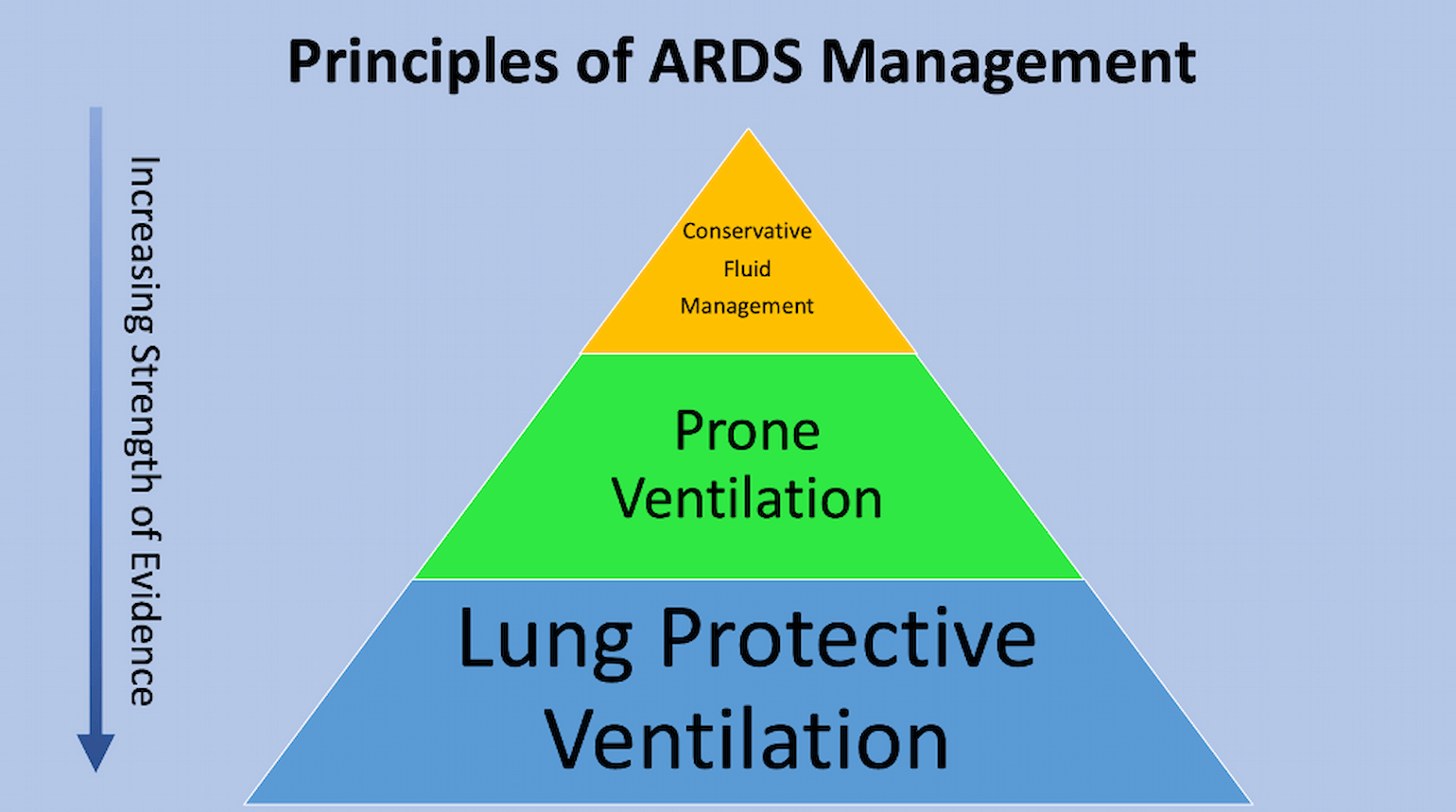
Figure 1
Principles of ARDS management (courtesy of Dr. C. Corey Hardin).
What is the Relationship Between Intravascular Volume and Pulmonary Edema in ARDS?
Conservative fluid management is one of the key principles of ARDS management (Figure 1). ARDS is a form of non-cardiogenic pulmonary edema, in which extravascular lung water increases due to increased pulmonary capillary permeability (Casey et al., 2019). As first articulated by Starling, fluid balance across the pulmonary capillary wall is related both to the hydrostatic and oncotic pressure differences between the intravascular and interstitial spaces (Sibbald et al., 1983) as well as to the permeability of the capillary wall (Figure 2).
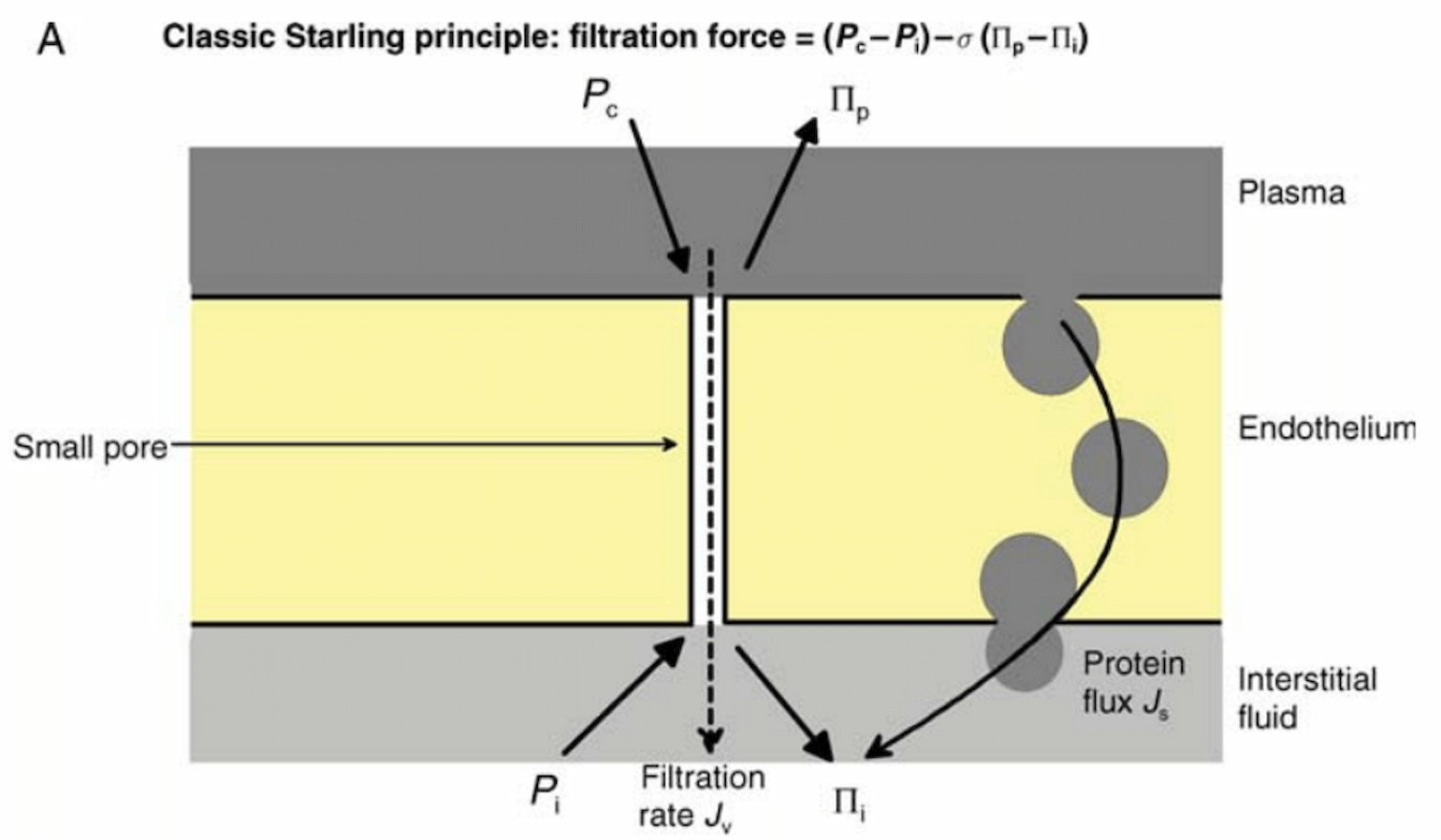
Figure 2
Starling’s view of fluid balance across the capillary wall. The filtration force of fluid into the pulmonary interstitium is defined by the hydrostatic pressure gradient between the pulmonary capillary (Pc) and interstitium (Pi) minus the oncotic pressure gradient of the plasma (Πp) and interstitium (Πi). The latter is multiplied by Staverman’s osmotic reflection coefficient, shown here as σ, which describes the degree of leakiness to a specific solute. Note that the oncotic pressure gradient is not determined solely by the concentration of intravascular proteins, as there are significant numbers of macromolecules in the interstitium. In part this is due to the endothelium slowly leaking (Js) proteins into the interstitial space.
Accordingly, attempts to minimize intravascular pressures could plausibly decrease edema formation in ARDS. The Starling model is likely oversimplified (ignoring the role of the glycocalyx, for example) and likely overstates the degree of resorption of fluid when intravascular oncotic pressure is high (Levick and Michel, 2010). Nevertheless, it is a useful approximation and it is because of these principles that we focus on fluid management in ARDS.
A Conservative Fluid Strategy is Recommended in ARDS, But Why, and What is That Exactly?
Both the Society of Critical Care Medicine and MGH critical care guidelines agree that those with COVID-19-associated ARDS should be treated with a conservative fluid strategy (Alhazzani et al., 2020). This recommendation is based on the results of the Fluid and Catheter Treatment Trial, or FACTT (ARDS Clinical Trials Network, 2006).
The FACTT Trial
As described above, in acute lung injury, pulmonary edema results from an increase in pulmonary capillary permeability, which can be exacerbated by any increase in hydrostatic forces. It follows that reducing intravascular volume might reduce edema, and thereby improve lung function. However, in ARDS, multiorgan failure rather than refractory hypoxemia is the most common cause of death (Stapleton et al., 2005; Villar et al., 2011). Accordingly, attempts to reduce edema must be balanced with the need for adequate resuscitation to prevent non-pulmonary organ failure.
FACTT Study Details
Methods:
Multi-center randomized study of 1,000 intubated patients with acute lung injury (a term no longer in use) as defined as P:F < 300, bilateral opacities on chest x-ray consistent with pulmonary edema, and no evidence of left atrial hypertension. Patients were assigned to either a liberal or conservative fluid strategy and followed for seven days. The FACTT trial had a complicated methodology owing to its two-by-two design, which simultaneously tested the utility of pulmonary artery catheters for guiding fluid management AND a conservative fluid strategy. Briefly, those in the liberal strategy group received either furosemide or fluids to move their intravascular pressure towards a goal of CVP 10-14 / PAOP 14-18, modified based on the use of vasopressors, signs of ineffective circulation and oliguria.
Of note, FACTT did not incorporate the use of diuretics for those in shock.
Results:
To summarize, in the conservative vs. liberal strategy groups, there was:
- No difference in the primary outcome of 60-day mortality (25.5% vs. 28.4%).
- A significant difference in cumulative fluid balance over seven days (-136 + 491 mL vs. 6992 + 502 mL).
- Improved oxygenation index, lung injury scores, and number of ventilator free days (14.6 vs. 11.2) in the conservative strategy group.
- Similar rates of renal replacement therapy use during the first 60 days (10% vs 14%, p=0.06).
- No increase in the incidence or prevalence of shock between groups.
Readers should note that FACTT relied on central venous pressures and pulmonary artery catheters to guide fluid or diuretic administration. It is no longer common to place pulmonary artery catheters in the MICU. Further, absolute values of central venous pressures do not correlate with volume responsiveness so may not be a sensitive or specific guide to volume status.
What data should guide fluid management (Marik and Cavallazzi, 2013)? An even simpler description of the FACTT conservative fluid strategy may be defined as:
When shock-free:
- No maintenance fluids
- Diuretics to normalize volume status until off the ventilator, as tolerated
- Hold diuretics for rising creatinine and/or active urine sediment
- If patient becomes hypotensive with small increases in PEEP, consider hypovolemia
Should Enteral Intake Count Towards Total Body Fluid Balance? An Unintended Lesson from FACTT
In the FACTT trial, fluid balance included enteral intake and may help us answer this question.
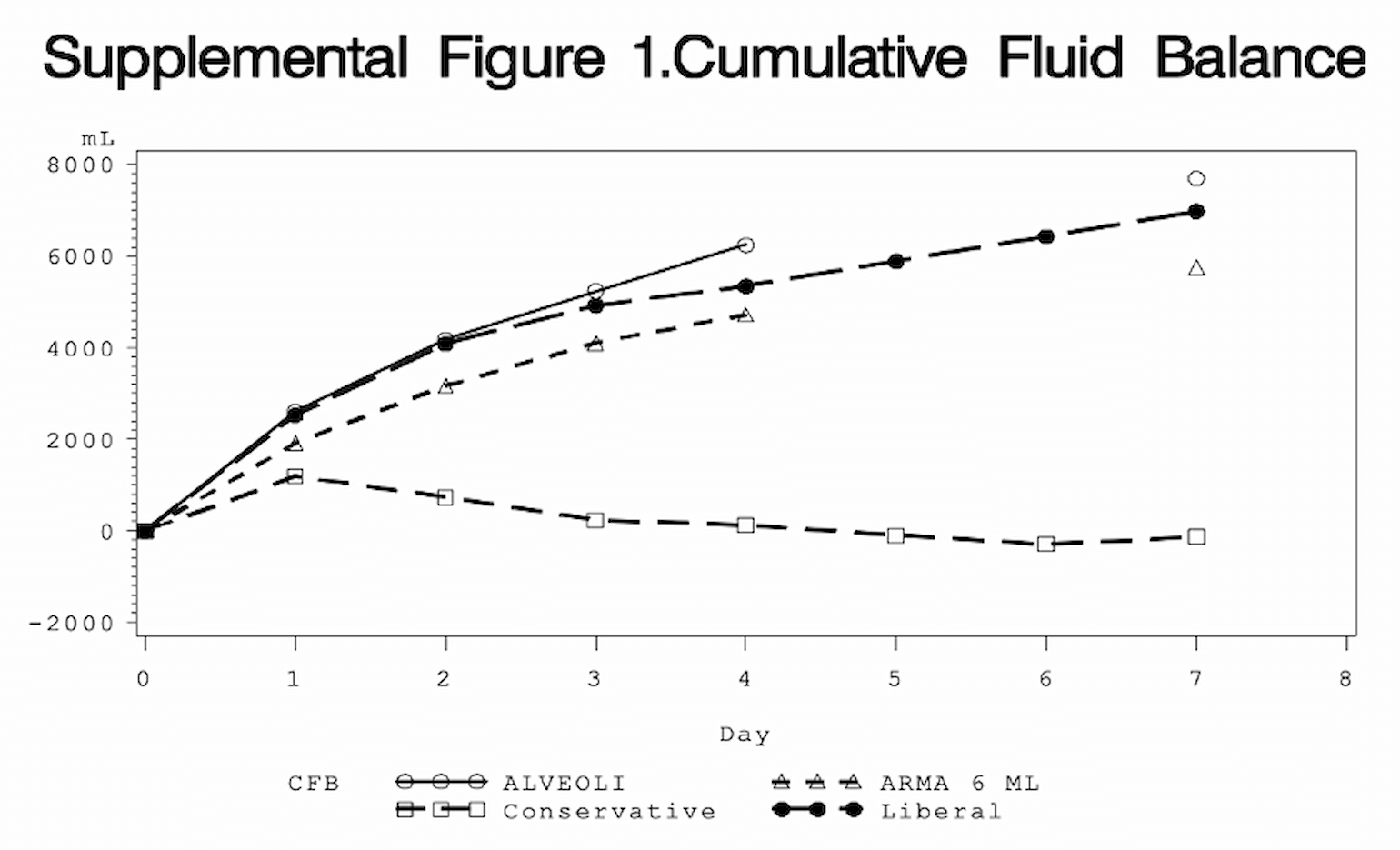
Figure 3
Comparison of cumulative fluid balance in the conservative (open squares) and liberal (black circles) arms of the FACTT trial. Also included are data from ARMA (RCT of 6 cc/kg vs. 12 cc/kg Vt in ARDS) and ALVEOLI (high vs. low PEEP in ARDS) trials.
Shown above is the mean cumulative fluid balance for the conservative and liberal groups across seven days. As you can see, the conservative group (open squares) was near even, while the liberal group (black circles) continued to accumulate fluid.
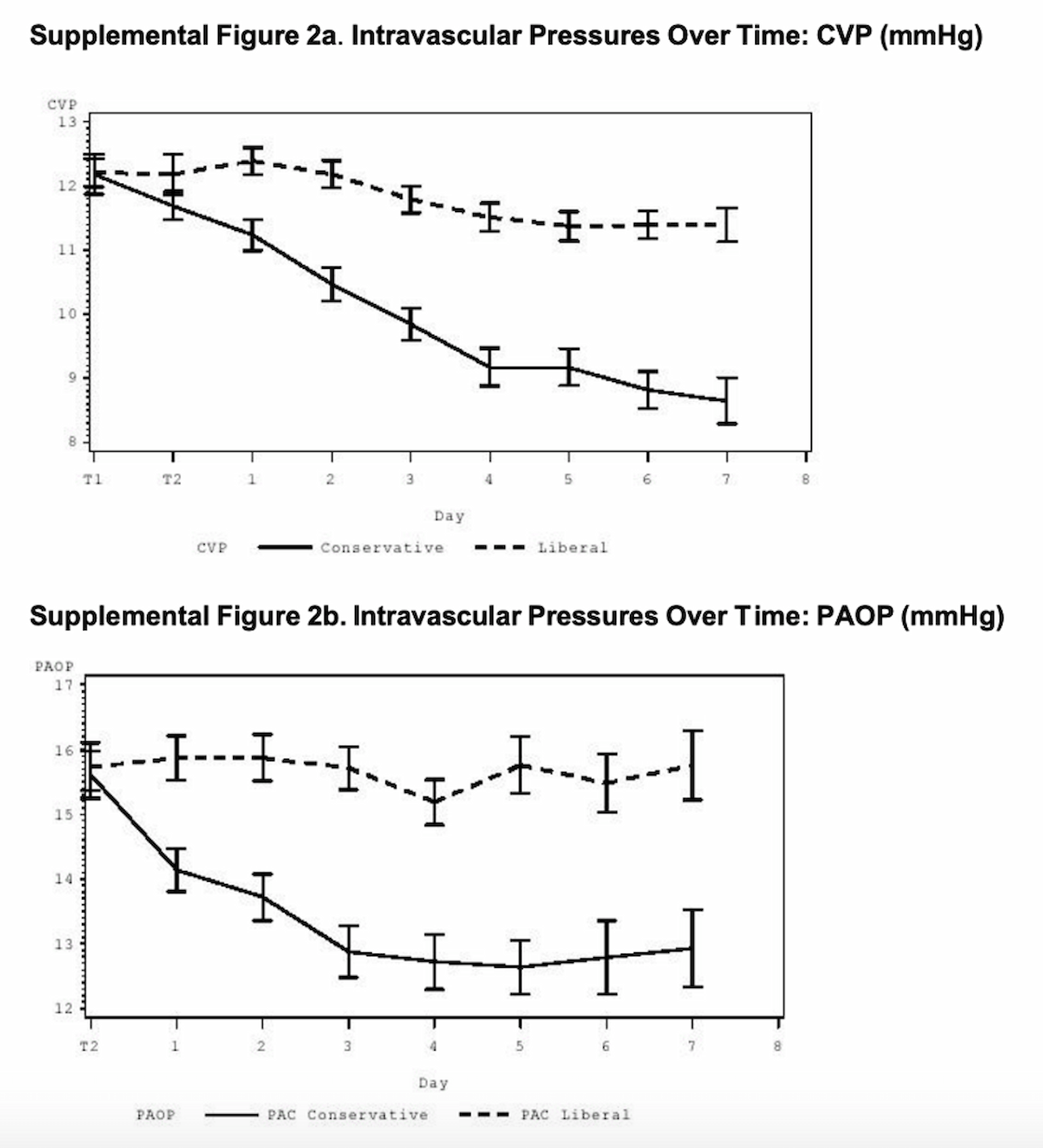
Figure 4
Despite having a near even cumulative fluid balance, in the conservative group, CVP and PAOP values trended down. This suggests that the conservative group was experiencing a decrease in intravascular volume (hence the change in CVP/PAOP) but their total fluid balance was interpreted as even because of enteral intake. The liberal group had a less profound change in CVP/PAOP, but according to cumulative fluid balance became net positive (presumably from recorded enteral intake). These data therefore suggest that enteral fluid intake may not have a substantial effect on intravascular volume.
How Should I Gauge Fluid Responsiveness?
Fluid responsiveness is most appropriately defined as an increase in stroke volume with an increase in venous return. As such, it depends on the shape of the cardiac starling curve at the point of its intersection with the venous return curve. The determination of the shape of a curve (its slope) requires knowledge of at least two points on that curve. For this reason, dynamic measures (with two data points, such as pulse pressure variation or passive leg raise) are preferred over static measures (which have a single data, such as CVP) to gauge volume responsiveness (Bednarczyk et al., 2017).
Summary
Both the MGH and the SCCM guidelines recommend conservative fluid management in COVID-19 patients. If a patient has been adequately resuscitated and does not have ongoing shock, diuresis can be initiated, with ongoing careful attention to volume status using validated methods. This strategy was demonstrated in the FACTT trial to increase ventilator-free days, though did not impact survival. In the era of COVID-19, this is not a trivial outcome as days off the ventilator can translate to another patient being able to utilize this resource. Thus, we agree with what many people are saying: dry (COVID-19) lungs are happy lungs.
References
- Alhazzani, W., Møller, M.H., Arabi, Y.M., Loeb, M., Gong, M.N., Fan, E., Oczkowski, S., Levy, M.M., Derde, L., Dzierba, A., et al. (2020). Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Medicine.
- ARDS Clinical Trials Network (2006). Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, DeBoisblanc B, Connors AF Jr, Hite RD, Harabin AL: Comparison of two fluid-management strategies in acute lung injury. N. Engl. J. Med. 354, 75.
- Bednarczyk, J.M., Fridfinnson, J.A., Kumar, A., Blanchard, L., Rabbani, R., Bell, D., Funk, D., Turgeon, A.F., Abou-Setta, A.M., and Zarychanski, R. (2017). Incorporating Dynamic Assessment of Fluid Responsiveness Into Goal-Directed Therapy. Critical Care Medicine 45, 1538–1545.
- Casey, J.D., Semler, M.W., and Rice, T.W. (2019). Fluid Management in Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 40, 57–65.
- Levick, J.R., and Michel, C.C. (2010). Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 87, 198–210.
- Marik, P.E., and Cavallazzi, R. (2013). Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit. Care Med. 41, 1774–1781.
- Sibbald, W.J., Warshawski, F.J., Short, A.K., Harris, J., Lefcoe, M.S., and Holliday, R.L. (1983). Clinical Studies of Measuring Extravascular Lung Water by the Thermal Dye Technique in Critically III Patients. Chest 83, 725–731.
- Stapleton, R.D., Wang, B.M., Hudson, L.D., Rubenfeld, G.D., Caldwell, E.S., and Steinberg, K.P. (2005). Causes and timing of death in patients with ARDS. Chest 128, 525–532.
- Villar, J., Blanco, J., Añón, J.M., Santos-Bouza, A., Blanch, L., Ambrós, A., Gandía, F., Carriedo, D., Mosteiro, F., Basaldúa, S., et al. (2011). The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 37, 1932–1941.
View all COVID-19 updates
Learn about research in the Division of Pulmonary and Critical Care Medicine