Update on Remdesivir
The FLARE Four
- Remdesivir is a nucleoside analog, previously studied in Ebola, which may have activity against SARS-CoV-2
- A recent paper in the NEJM reported the clinical course of COVID-19 patients treated with remdesivir through compassionate use protocols
- This industry-sponsored study was uncontrolled and rife with methodological issues
- Evidence on the effectiveness of remdesivir will require additional trials. Fortunately, many such trials are ongoing
Many people are asking...wasn't there a new trial of remdesivir published?
Subscribe to the latest updates from FLARE Advances in Motion
What is the Rationale for the Use of Remdesivir in SARS-CoV-2 Infection?
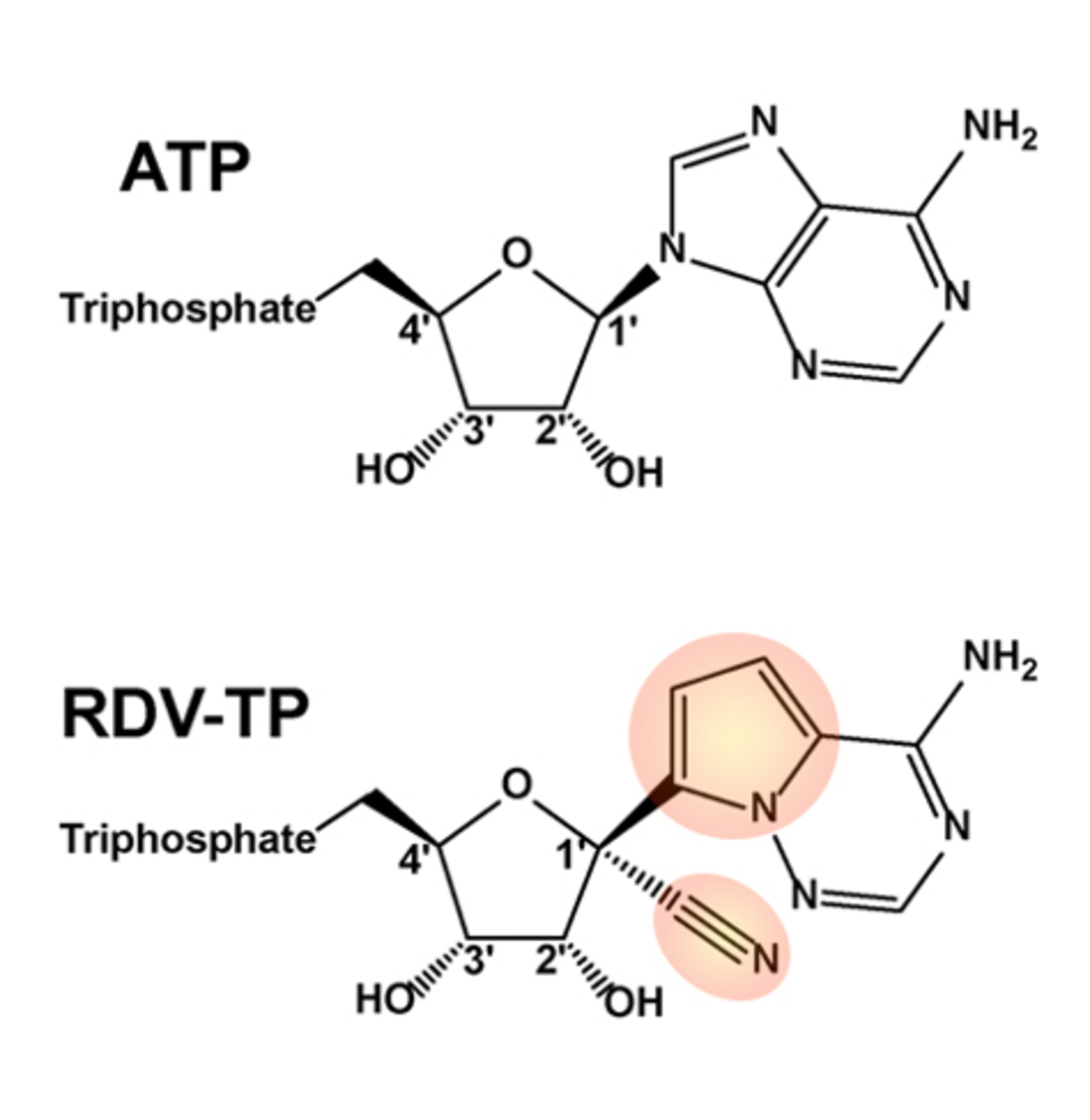
Figure 1
Figure 1: chemical structures of remdesivir triphosphate and ATP. Highlighted portions are those which differ from ATP (ASBMB).
Molecular Biology and Biochemistry
Remdesivir (RDV), a nucleoside analog of adenosine, exhibits wide-ranging anti-viral properties in several viral families by inhibiting RNA polymerases. Although SARS-CoV and SARS-CoV-2 share only 82% RNA sequence identity, their RNA-dependent RNA polymerases (RdRp) share 96% sequence identity. Therefore, drugs targeting viral RdRp proteins of SARS-CoV are likely to be effective for SARS-CoV-2. Remdesivir has shown in vitro activity against SARS-CoV (Sheahan et al. 2017, Agostini et al. 2018), but has yet to demonstrate efficacy in human SARS-CoV. As a prodrug, remdesivir is metabolized by host cells to produce remdesivir triphosphate (RDV-TP), an analog of ATP, which differs primarily by the presence of a 1’ cyano group (Figure 1). RDV-TP is incorporated by viral RNA polymerases into nascent transcripts, resulting in premature chain termination and halting viral replication.
At first glance, it is not obvious that Coronaviruses like SARS-CoV-2 would be highly susceptible to RDV as these viruses encode a “proofreading” 3’->5’ exonuclease, which would be expected to excise the newly incorporated drug from transcripts. In fact, this proofreading activity underlies SARS’ and MERS’ near-complete resistance to inhibition by the guanosine analog ribavirin (Ferron et al. 2018). However, recent work performed with the MERS-CoV RdRp has shed light on why RDV might be uniquely poised as an anti-Coronaviral agent (Gordon et al. 2020). This study indicates that RDV has an interesting mechanism of “delayed chain termination” wherein several additional nucleotides are incorporated after RDV (a feature dependent on the presence of a 3’ hydroxyl group, as depicted above); these extra bases may protect RDV from excision by the proofreading exonuclease and thereby increase the viral inhibitory effects of the drug. In addition, RDV-TP is actually preferred to ATP by MERS-CoV’s polymerase, suggesting it might be effective at relatively low concentrations.
Published Data for Use in SARS-CoV-2
In vitro data (Wang et al. 2020) indicate that remdesivir potently blocks SARS-CoV-2 infection at low micromolar concentrations and with a high selectivity index (a measure of anti-viral versus cytotoxicity effect). Furthermore, the EC90 of remdesivir in this study can likely be achieved in non-human primates based on prior data using remdesivir in Ebola studies. During the COVID-19 pandemic, there have been a small number of case reports published using remdesivir.
In vivo, remdesivir has been primarily studied in Ebola Virus Disease (Mulangu et al. 2019). Briefly, remdesivir was included as one of four therapies in a randomized trial of patients with confirmed Ebola virus disease. A total of 175 patients received the drug, but during interim analysis, randomization to the remdesivir group was halted in favor of two other agents. However, the remdesivir group did have reduced mortality in comparison to the control group (3.4%, CI -7.2 to 14.0), though this was not statistically significant. Although other agents are favored for the treatment of Ebola Virus Disease, the safety data for remdesivir were acceptable.
In light of the above in vitro and in vivo data, remdesivir was an attractive option for treatment of SARS-CoV-2 infection, and was made available through compassionate use protocols and clinical trials.
What About That New Trial?
Compassionate Use of Remdesivir for Patients with Severe COVID-19 (Grein et al. 2020)
Remdesivir was provided via compassionate use protocols to 53 patients with documented SARS-CoV-2 infection who had:
- Oxygen saturation of 94% or less on room air or who were requiring supplemental oxygen
- Creatinine clearance > 30ml/min
- Serum ALT and AST < 5X the upper limit of normal
- Not receiving other agents for treatment of SARS-CoV-2
Methodology
All patients were assigned to a 10-day course of remdesivir (200 mg on day 1, then 100 mg daily for the following 9 days). Patients were followed for at least 28 days or until discharge or death. Patients were evaluated for clinical improvement as defined by discharge from hospital and/or decrease in score by two points on a six-point ordinal scale (1 = not hospitalized; 2 = hospitalized, not on O2; 3 = hospitalized, on HFNC or NIPPV; 4 = hospitalized, mechanically ventilated or ECMO; 6 = death).
What Were the Results?
Patient Characteristics
Briefly, 53 patients had sufficient data for inclusion in the study, 40 (75%) of whom received the full 10-day course of remdesivir. Respiratory support data are included in the table below.Importantly, the median duration of symptoms before the initiation of remdesivir treatment was 12 days (IQR 9-15).
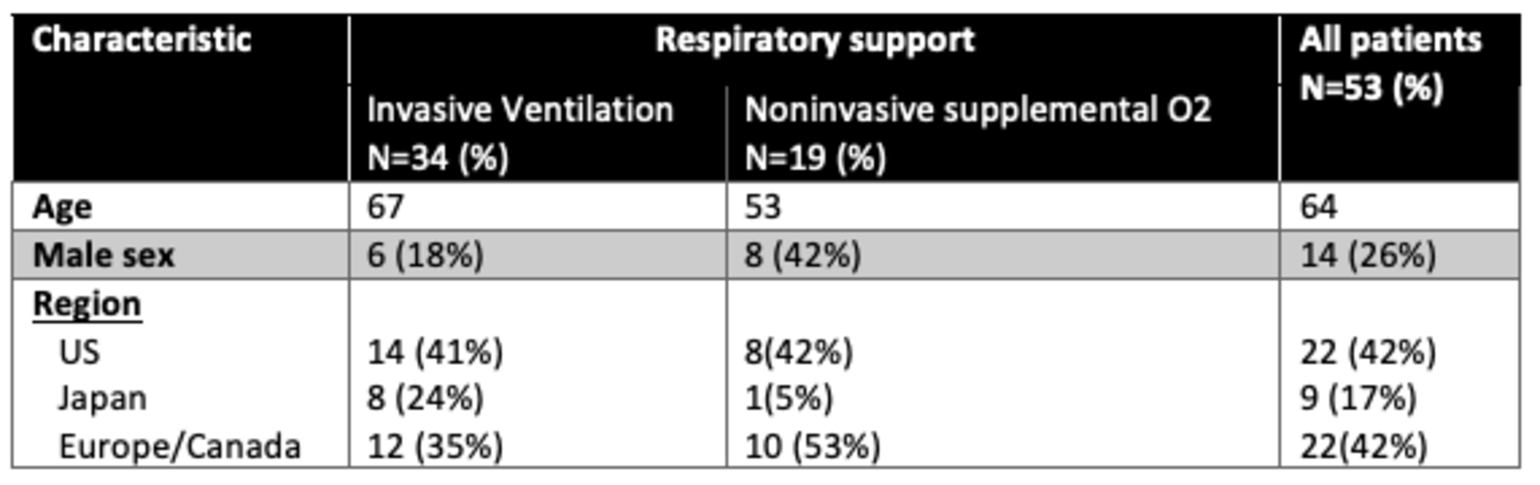
Table 1
Baseline characteristics of the trial patients.
Outcomes
Improvement in oxygen support: 36/53 (68%)
- In those on ambient air or low flow supplemental O2: 12/12 (100%)
- In those on HFNC or NIPPV: 5/7 (71%)
- In those on mechanical ventilation: 17/30 extubated (57%)
- In those on ECMO: 3/4 (75%)
Mortality, overall: 7/53 (13%) after completion of the remdesivir course.
Adverse Events
In general, remdesivir was well-tolerated with an acceptable number of adverse events.
- Hepatic enzyme elevation: 12/53 (23%)
- Diarrhea: 5/53 (9%)
- Rash: 4/53 (8%)
- Renal impairment: 4/53 (8%)
- Hypotension: 3/53 (8%)
- Serious adverse events: 12/53 (23%), including MODS (2/53, 4%), septic shock (2/53, 4%), AKI (2/53, 4%) and hypotension (2/53, 4%)
Criticisms
This study does not provide meaningful information on the effectiveness of remdesivir for several reasons. There is no randomization or a control group. It is possible that this study simply details the natural history of SARS-CoV-2 infection. Patients received variable courses of treatment, starting usually later on in the course of infection. There is no information provided on the effect of remdesivir on viral load, which would at least establish some plausibility to the claimed benefits. There was no predefined stopping point for enrollment. Furthermore, we cannot determine the rate of adverse events secondary to therapy given that there is not a comparative cohort that did not receive the drug. Additionally, this was a compassionate use study - patients only received the drug after applying to Gilead for it. No information is provided on how these patients were selected or rejected for therapy. The paper was written and edited with assistance from Gilead employees, which demonstrates a conflict of interest. Given all of these concerns, the study results cannot be used to guide clinical decision making.
Can We Conclude Anything About This Uncontrolled Trial by Using Controls from Other Trials?
In short: no. The authors of the remdesivir trial in question attempt to compare the mortality outcomes of the patients in their study to patients in a recently-published study of lopinavir-ritonavir open label RCT (Cao et al. 2020), with very similar inclusion criteria. However, this comparison is fraught with methodological problems. For example, the remdesivir trial enrolled internationally, whereas the lopinavir-ritonavir trial enrolled patients in one hospital. Furthermore, the size of the cohort in the remdesivir trial is very small (53 patients). Finally, inclusion through a compassionate use program introduces bias as we do not know why these particular patients were selected out of the many who applied for this program internationally. Others have come to similar conclusions.
In fact, as the authors themselves (Grein et al. 2020) state: “Interpretation of the results of this study is limited by the small size of the cohort, the relatively short duration of follow-up, potential missing data owing to the nature of the program, the lack of information on 8 of the patients initially treated, and the lack of a randomized control group.”
We agree.
Conclusion
Remdesivir is a plausible therapy for SARS-CoV-2 and should be tested in a rigorous way. The paper reviewed here is an industry-sponsored, uncontrolled, non-randomized study, which should not be used to guide clinical decision making. We await the results of a randomized and placebo-controlled clinical trial to determine the effect of remdesivir on patient outcomes.
References
- Agostini, Maria L., Erica L. Andres, Amy C. Sims, Rachel L. Graham, Timothy P. Sheahan, Xiaotao Lu, Everett Clinton Smith, et al. 2018. “Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease.” mBio 9 (2). https://doi.org/10.1128/mBio.00221-18.
- Cao, Bin, Yeming Wang, Danning Wen, Wen Liu, Jingli Wang, Guohui Fan, Lianguo Ruan, et al. 2020. “A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19.” The New England Journal of Medicine, March. https://doi.org/10.1056/NEJMoa2001282.
- Ferron, François, Lorenzo Subissi, Ana Theresa Silveira De Morais, Nhung Thi Tuyet Le, Marion Sevajol, Laure Gluais, Etienne Decroly, et al. 2018. “Structural and Molecular Basis of Mismatch Correction and Ribavirin Excision from Coronavirus RNA.” Proceedings of the National Academy of Sciences of the United States of America 115 (2): E162–71.
- Gordon, Calvin J., Egor P. Tchesnokov, Joy Y. Feng, Danielle P. Porter, and Matthias Götte. 2020. “The Antiviral Compound Remdesivir Potently Inhibits RNA-Dependent RNA Polymerase from Middle East Respiratory Syndrome Coronavirus.” The Journal of Biological Chemistry 295 (15): 4773–79.
- Grein, Jonathan, Norio Ohmagari, Daniel Shin, George Diaz, Erika Asperges, Antonella Castagna, Torsten Feldt, et al. 2020. “Compassionate Use of Remdesivir for Patients with Severe Covid-19.” The New England Journal of Medicine, April. https://doi.org/10.1056/NEJMoa2007016.
- Mulangu, Sabue, Lori E. Dodd, Richard T. Davey Jr, Olivier Tshiani Mbaya, Michael Proschan, Daniel Mukadi, Mariano Lusakibanza Manzo, et al. 2019. “A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics.” The New England Journal of Medicine 381 (24): 2293–2303.
- Sheahan, Timothy P., Amy C. Sims, Rachel L. Graham, Vineet D. Menachery, Lisa E. Gralinski, James B. Case, Sarah R. Leist, et al. 2017. “Broad-Spectrum Antiviral GS-5734 Inhibits Both Epidemic and Zoonotic Coronaviruses.” Science Translational Medicine 9 (396). https://doi.org/10.1126/scitranslmed.aal3653.
- Wang, Manli, Ruiyuan Cao, Leike Zhang, Xinglou Yang, Jia Liu, Mingyue Xu, Zhengli Shi, Zhihong Hu, Wu Zhong, and Gengfu Xiao. 2020. “Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-nCoV) in Vitro.” Cell Research 30 (3): 269–71.
View all COVID-19 updates
Learn more about the Division of Pulmonary and Critical Care Medicine