Making CAR T-Cell Therapy More Effective Against Solid Tumors
In This Article
- Chimeric antigen receptor (CAR) T-cell therapy has changed the treatment paradigm in hematologic cancers but has not been successful against solid tumors
- Massachusetts General Hospital researchers have developed a novel way to ready the tumor microenvironment for CAR T-cell therapy and make CAR T-cells more effective in solid tumors
- Because the interventions are already approved and safe for clinical use, the approach could be implemented quickly in patients with solid tumors
Massachusetts General Hospital researchers have developed a novel way to increase the effectiveness of chimeric antigen receptor (CAR) T-cell therapy against solid tumors.
Subscribe to the latest updates from Oncology Advances in Motion
"CAR T-cell therapy has revolutionized and achieved unprecedented success in treating some blood cancers by genetically modifying T-cells to target and eliminate cancer cells. However, the results of CAR T-cell therapy in solid tumors have been generally disappointing," says Xinhui Wang, MD, PhD, principle investigator in surgical oncology at the Mass General Research Institute. "Our study offers a timely innovation to amplify CAR T-cell therapy's effectiveness against solid tumors."
CAR T-Cell Therapy's Limited Effectiveness in Solid Tumors
CAR T-cell therapy has been a major paradigm shift in treating several hematologic cancers, but researchers have struggled to translate that success into the treatment of solid tumors. Clinical success of CAR T-cell therapy depends on engineered T-cells surviving, multiplying, and then developing memory T-cells to achieve durable responses. However, CAR T-cells have been unable to adequately infiltrate solid tumors, multiply, or persist, and the tumor microenvironment has immunosuppressive properties (referred to as cold tumors) preventing T-cells from working effectively.
Although significant efforts have been made to further genetically engineer CAR T-cells to overcome these obstacles, they have had limited success, Dr. Wang says. Furthermore, genetic modifications come with potential risks of off-target effects by inadvertently targeting normal tissue, adds Genevieve Boland, MD, PhD, vice chair of research in the Department of Surgery at Mass General.
Dr. Wang was confident that she could use already approved interventions to stress cancer cells and amplify CAR T-cell therapy's effectiveness without modifying the genetic makeup of CAR T-cells. The idea was based on previous research from the Wang Laboratory that found that disulfiram (DSF), a drug approved by the FDA for treating alcohol dependence, chelates with copper (Cu), an essential nutrient, to form DSF/Cu complexes. DSF/Cu can induce immunogenic cell death, especially when combined with radiation. She was confident that the dual approach (as a stressor) could reprogram the CAR T-cells with acquired early memory T or juvenile cell characteristics and tumor microenvironment from "cold" to "hot" with infiltrated immune and CAR T-cells and help overcome the barriers to CAR T-cell therapy's success against solid tumors.
Comparing Stressed Versus Nonstressed CAR T-Cell Therapy
The research team tested their theory in mice. One group received CAR T-cell therapy alone. In the other group, the researchers administered an intralesional injection of DSF/Cu the first day, ionizing radiation (IR) the second, and CAR T-cell therapy the third day.
"This novel approach focuses on the host, the recipient of the CAR T-cells, and how we can modify the tumor to be more receptive. And it actually trains the CAR T-cells in vivo to be more successful," says Dr. Boland. "It primes the host to get the most beneficial and durable response through memory T-cell formation for a sustained effect."
After treatment, the investigators analyzed blood and tissue samples to determine the number of CAR T-cells that had infiltrated tumor tissues, as well as levels of pro-inflammatory cytokines and chemokines. They found that CAR T-cells in the treatment group had more potent cytotoxicity, enhanced ability to proliferate, and memory characteristics. The intervention also reversed the immunosuppressive qualities of the tumor microenvironment.
"To our surprise, the tumor simply disappeared in the treatment group. CAR T-cell therapy alone usually gets only 50% tumor reduction, but in the stressor and CAR T-cells treated group, we found that the tumors all disappeared," Dr. Wang says. Remarkably, these mice exhibited a strong memory response against solid tumors: 60% remained tumor-free even after two subsequent reinoculations of the same tumor cells (rechallenges) throughout the duration of the experiment, lasting until day 300. Regardless of CAR T-directed targets, similar preclinical outcomes were obtained in multiple mouse tumor models, e.g., patient-derived xenograft (PDX) and human pancreatic cancer cell line-derived tumors.
A Turning Point for CAR T-Cell Therapy in Solid Tumors
Dr. Wang's team then explored whether they could duplicate this effect using CAR T-cells derived from 20 patients with triple-negative breast cancer, which is notoriously difficult to treat.
Again, they established two groups of mice: one receiving CAR T-cell therapy alone and the other receiving CAR T-cell therapy plus the stressor. In the stressor group, eight of 20 mice had a complete response, and the remaining 12 had a partial response. Compared to the control group, the mice in the stressor group experienced better reduction of tumor volume, more in vivo CAR T-cell expansion, and creation of early memory T-cells.
"The introduced stress on cancer cells or tumors induces communication between the cancer cells and the CAR T-cells through biological signaling. This interaction transforms the CAR T-cells, endows them with early memory cell-like attributes, and strengthens their capacity to eliminate cancer cells," Dr. Wang says. "Additionally, this approach reprograms the tumor microenvironment and renders it favorably 'hot' instead of a 'cold' environment for tumor infiltration and function of CAR T-cells."
The researchers believe the findings are a turning point in the application of CAR T-cell therapy, potentially providing enduring cures across various solid tumor types. The study was recently published in Nature Communications.
Translating Findings to Clinical Care
Now that the lab has established proof-of-concept, Drs. Wang and Boland hope the approach can quickly and easily be translated into clinical practice through the right partnership.
"The barrier to entry with this approach is relatively low because we would not be trying a new, untested drug. We are repurposing existing agents. These are approved interventions that have a good toxicity profile and are not costly," Dr. Boland explains.
They hope an existing CAR T-cell research group or sponsor will add an arm to an existing standard-of-care study.
A Tradition of Collaboration and Persistence
Drs. Boland and Wang credit this potential breakthrough to the longstanding collaborative and persistent culture at Mass General.
"One of our strengths is that we use clinical trials not only to look at efficacy or safety but also to understand biology. Embedded within all of our clinical trial programs is deep correlative research. So when we get good results, we're happy, but when we get the results that we don't hope for, we can understand why, and that helps us iterate the next generation of research to overcome those barriers," Dr. Boland says.
Dr. Wang also acknowledges the contributions of Mass General's Soldano Ferrone, MD, PhD, a pioneering cancer immunologist who was a mentor, colleague, friend, and collaborator. These new findings stem from their years working together developing antibodies and CAR T-cell therapies.
Scroll to View Figures 1a-d
Robust and Long Sustained Therapeutic Responses Against PDX by DSF/Cu+IR +B7-H3 CAR T Derived From PBMCs of a Patient With Metastatic Breast Cancer:
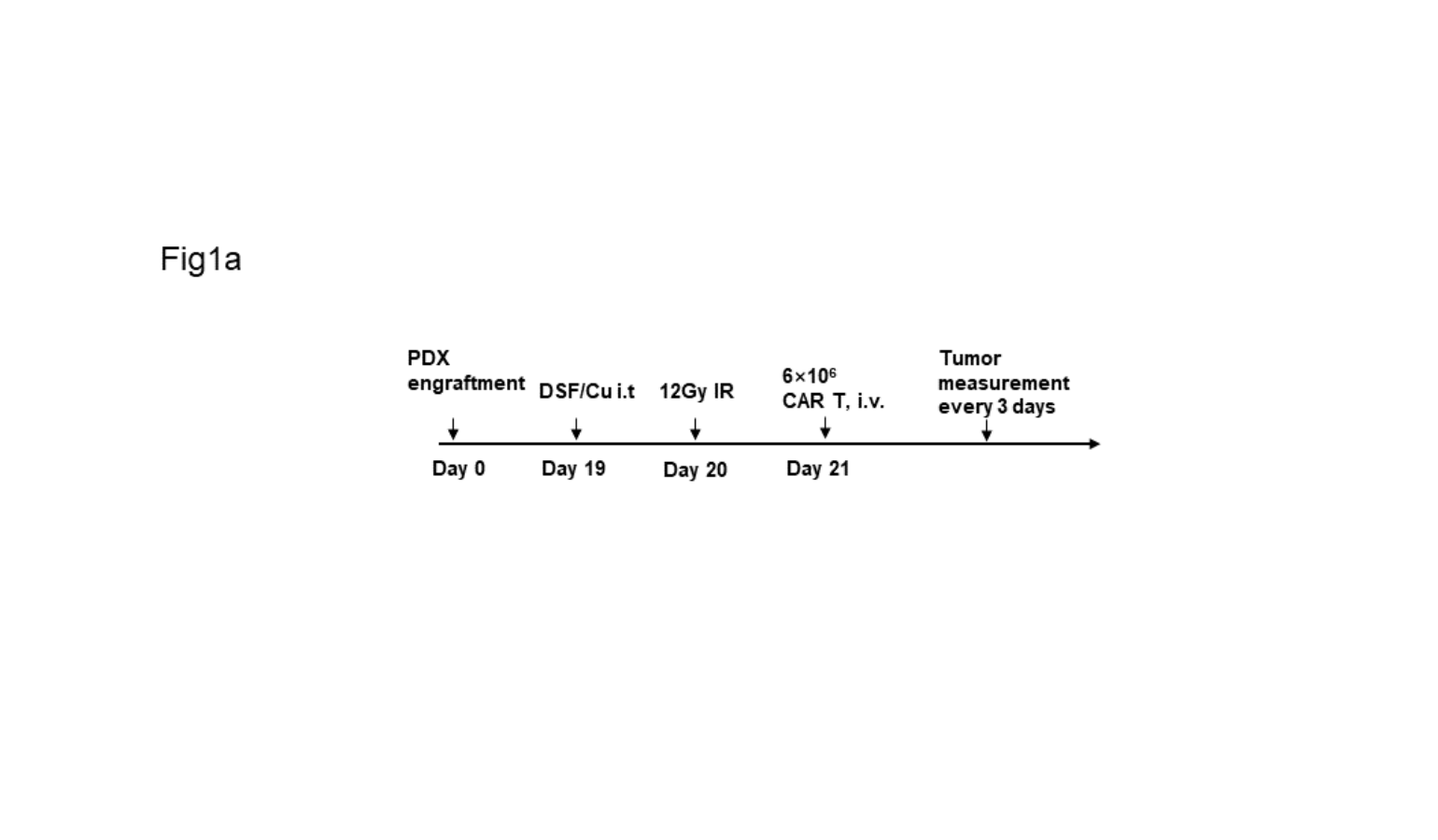
Figure 1a
Schema of TNBC-PDX mouse model. Image courtesy of Xinhui Wang, MD, PhD.

Figure 1b
Tumor volumes (Untreated= 6; the other two groups n = 7) of each group treated either with B7-H3 CAR T only (tumor reduction was obtained compared to that in untreated group) or DSF/Cu+IR + B7-H3 CAR (complete tumor rejection was achieved in 100% mice). The B7-H3 CAR T cells were derived from the same patient: a 75-year-old individual with metastatic TNBC. Image courtesy of Xinhui Wang, MD, PhD.

Figure 1c
Frequency of B7-H3 CAR T cells (CD3+CD45+) in the blood of mice collected weekly (n = 7/group). Image courtesy of Xinhui Wang, MD, PhD.

Figure 1d
Kaplan–Meier survival curve of mice: 100% remained tumor-free throughout the duration of the experiment, lasting until day 250, while CAR T only treated mice were all dead before day 150. Image courtesy of Xinhui Wang, MD, PhD.
Learn more about CAR T-cell therapies
Learn more about the Mass General Cancer Center