Obstetric Units Can Be Used Efficiently As Remdesivir Infusion Sites for Pregnant and Postpartum Outpatients
Key findings
- Pregnant individuals with COVID-19 are at increased risk of severe disease, hospitalization, ICU admission, and death
- In early 2022, at a time of medication shortages and limited resources for IV medication therapy, providers created a protocol to use labor and delivery units for administering remdesivir to high-risk pregnant and postpartum outpatients with COVID-19
- Eight patients received intravenous remdesivir as outpatients on Labor and Delivery; none progressed to severe COVID-19, and no maternal or neonatal adverse events occurred
- Obstetric units should create a protocol to offer remdesivir to pregnant patients with mild COVID-19 as future constraints on Nirmatrelvir–ritonavir, Paxlovid (drug-drug interactions), and monoclonal antibody (resistant strains) use can be anticipated
Monoclonal antibody treatment with sotrovimab or antiviral treatment with molnupiravir, nirmatrelvir–ritonavir, or remdesivir is recommended for outpatients with mild to moderate COVID-19 who have risk factors for severe disease. Pregnancy is one of those factors.
Subscribe to the latest updates from OB/GYN Advances in Motion
In early 2022, with the omicron variant of SARS-CoV-2 surging globally, physicians and nurses at Massachusetts General Hospital created a protocol to use labor and delivery (L&D) units to administer remdesivir to high-risk pregnant and postpartum outpatients who had mild-to-moderate COVID-19. Remdesivir was widely available by that time, but the need for intravenous administration of three consecutive daily doses posed significant hospital-wide logistical challenges because infusion beds and nurses were limited across the institution.
Ilona T. Goldfarb, MD, MPH, maternal–fetal medicine specialist in the Department of Obstetrics and Gynecology, and colleagues describe the protocol and the outcomes of eight patients in The Journal of Hospital Infection.
Eligibility
To minimize the impact of treating outpatients to the high-volume care of laboring patients, eligibility for this therapy was based on known risk factors. Patients were eligible for treatment if they were pregnant or postpartum (≤4 weeks) and had all of the following:
- A positive COVID-19 antigen or polymerase chain reaction test
- Mild-to-moderate COVID-19 symptoms for ≤7 days
- At least one other risk factor for severe COVID-19: unvaccinated or under-vaccinated (partial primary course or absence of booster dose); body mass index ≥35 kg/m2; diabetes requiring medication; severe cardiovascular disease; severe immunosuppression; chronic kidney, liver, or lung disease; or sickle cell disease
Outpatients were treated in L&D triage.
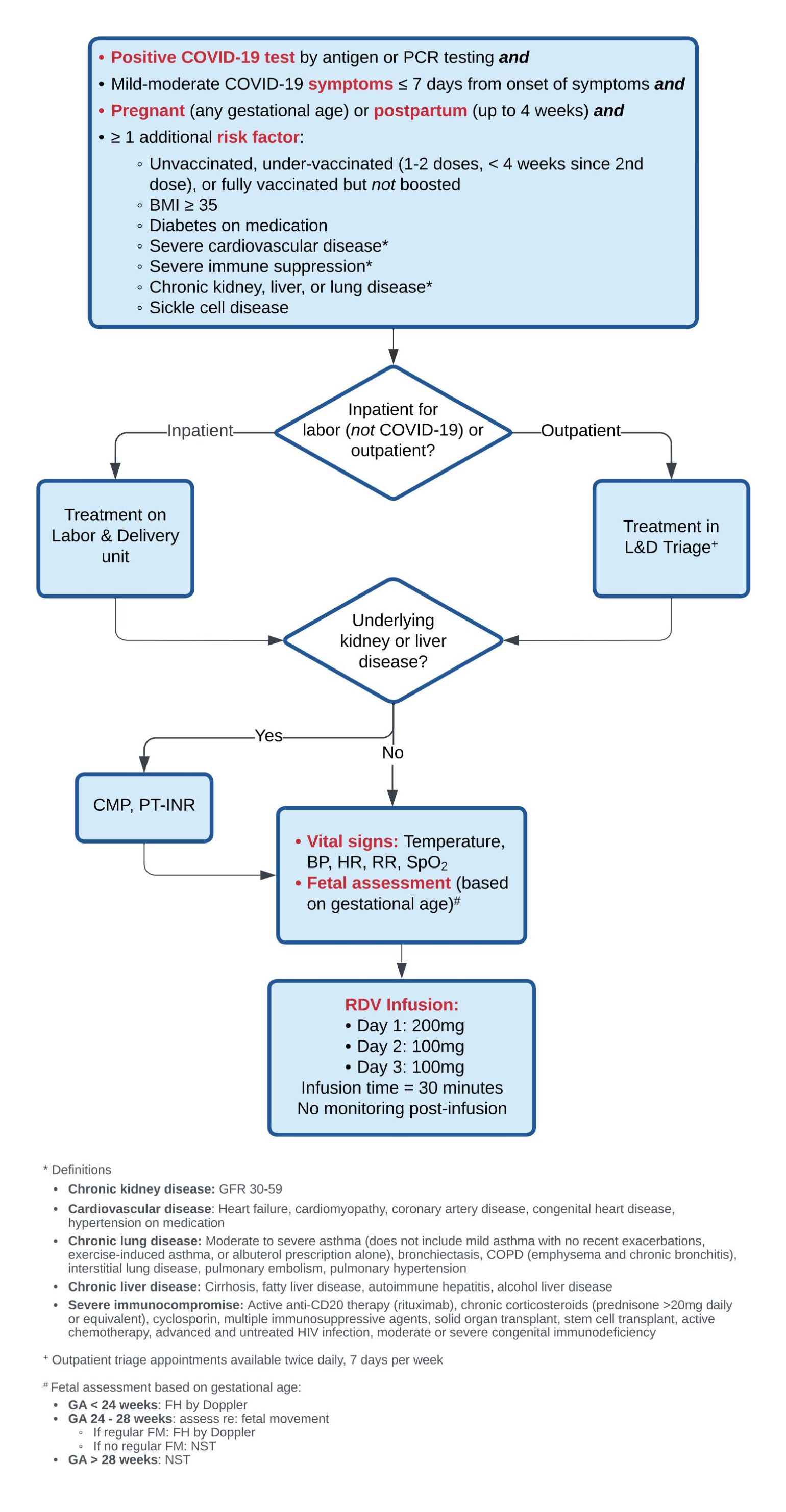
Figure 1
Remdemsivir Infusion Protocol designed by physicians and nurses at Mass General. Image courtesy of Ilona T. Goldfarb, MD, MPH.
Assessments
Pre-infusion labs were drawn only if the patient had known underlying liver or kidney disease (if indicated, complete metabolic panel and prothrombin time/international normalization ratio). Vital signs were monitored during the infusion, including temperature, blood pressure, heart rate, respiratory rate, and oxygen saturation.
The type of fetal assessment was based on gestational age:
- <24 weeks—Fetal heart rate was assessed by Doppler
- 24–28 weeks—If there was regular fetal movement, fetal heart rate was monitored by Doppler; otherwise, cardiotocography was performed
- >28 weeks—The fetus was monitored with cardiotocography
Infusions
30-minute infusions were given as 200 mg on day 1 and 100 mg on days 2 and 3. There was no post-infusion monitoring.
There was no extension of treatment beyond three consecutive days. Per protocol, if a patient did not return on day 2, the second dose would be given on day 3, and treatment would be considered complete.
Outcomes
Seven patients received all three doses of remdesivir and one patient received one dose. No patients progressed to having severe disease. No maternal or neonatal adverse events occurred.
Commentary
Pregnant individuals with COVID-19 are at increased risk of severe disease, hospitalization, ICU admission, and death, and as such, should be prioritized for medical management of mild to moderate COVID-19 infection to prevent progression to severe disease.
Though several options for outpatient treatment are now more readily available, Remdesivir should remain a viable alternative for every obstetrical practice. Nirmatrelvir–ritonavir (Paxlovid) may be more appealing as an oral therapy, but its many notable drug–drug interactions make it inappropriate for some high-risk pregnant patients. Monoclonal antibody therapy is less of a logistical challenge given only a single intravenous infusion is therapeutic. However, this type of therapy may be intermittently unavailable as it is subject to significant reductions in effectiveness with the development of new variants.
Remdesivir for COVID-19 has been evaluated in several observational studies, which suggest it is safe and well tolerated in pregnant and postpartum patients with COVID-19.
As obstetrical units have facilities and staff for intravenous infusions, Labor and Delivery units should create a protocol to offer outpatient remdesivir to pregnant patients with mild-to-moderate COVID-19.
view original journal article Subscription may be required
Learn more about Maternal-Fetal Medicine at Mass General
Refer a patient to the Maternal-Fetal Medicine Program at Mass General