Evaluating Post-Menopausal Women's Vulvovaginal Symptoms, Mood and Sex Life
In This Article
- A recent study of postmenopausal women assessed how much they were bothered by vulvovaginal discomfort symptoms, along with impact on mood and quality of life
- This pioneering study evaluate efficacy of placebo and two common, low-risk treatments: low-dose vaginal estradiol tablets and non-hormonal vaginal moisturizer
- Both hormonal and non-hormonal agents provided safe, easily available relief of vulvovaginal symptoms, which affect approximately 50% of postmenopausal women
- Estrogen preparations were associated with a slightly larger improvement in quality of life, indicating more research is needed to understand the full spectrum of impact of genitourinary syndrome of menopause
While all women with menopause experience a decrease in estrogen, only about 50% suffer moderate to severe vulvovaginal dryness, discomfort or pain with penetration. Sexual desire and quality of sex life tend to be negatively impacted by these symptoms.
Subscribe to the latest updates from OB/GYN Advances in Motion
The North American Menopause Society coined the term "genitourinary syndrome of menopause" (GSM) in 2014 to express the heterogeneous nature and prevalence of these symptoms. Coupled with the emergence of women's health as a formal clinical specialty and the expanding knowledge of human aging biology, the health concerns of postmenopausal women are now being investigated with more depth and rigor than ever before. At Massachusetts General Hospital, these efforts are being led by Caroline M. Mitchell, MD, MPH, a researcher with the Vincent Center for Reproductive Biology (VCRB).
The Need for Better Understanding of Vaginal Biology
"Our basic understanding of the biology of the vagina has, until recently, been very limited," explains Dr. Mitchell. "Studying vaginal biology is very different from studying other organs. There is not an animal model for the human vagina."
But research into postmenopausal women's symptoms is vital, she says, because one of the big takeaways from her group's work is how prevalent vulvovaginal distress is and how motivated postmenopausal women are to mitigate symptoms.
"What brings people to tears is sex," she says. "It's that they are so bothered by symptoms they can no longer be intimate with their partner. At the primary care level, I think providers should be asking postmenopausal women if they are having vaginal discomfort—and do they know there are easy things they can do about it."
Pioneering Menopause Studies
Dr. Mitchell led and collaborated on two recently published papers from a randomized trial that provides an evidence base to help guide improved treatments. She is the lead investigator in a study published in JAMA Internal Medicine and a co-author on a study in Menopause: The Journal of the North American Menopause Society.
The two papers report results from a randomized trial that was the first of its kind: large-sample (n=302), 12-week, randomized, double-blind, placebo-controlled trial to evaluate two common treatments for postmenopausal vulvovaginal symptoms in women (mean age was 61 years).
In the randomized trial, investigators studied two common treatments and placebo:
- Low-dose (10 µg) estradiol tablets plus placebo gel (n=102)
- Placebo tablet plus vaginal moisturizer (n=100)
- Dual placebo tablet and moisturizer (n=100)
The JAMA report evaluated the effects of interventions on bothersome symptoms (figure 1). Symptoms included moderate to severe postmenopausal vulvovaginal itching, pain, dryness and irritation or pain with penetration. The Menopause study used data from the same 302 participants, but focused on assessing the impact of symptom relief on mood and quality of life (figure 2).
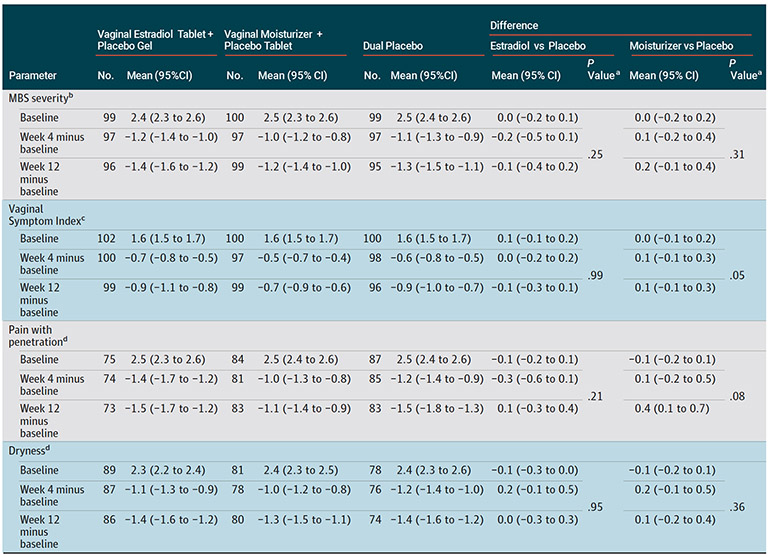
Fig. 1: Most Bothersome Symptom (MBS) Severity
Chart shows reported MBS severity at week 4 and week 12 of treatment for the 302 postmenopausal women included in the study.
Notes: a - P values from comparison of each treatment vs placebo in a repeated measures linear model of outcome as a function of randomization assignment, baseline value of the outcome measure, visit week (categorical), and clinical site.
b - Participants scored vulvovaginal itch, pain, dryness, irritation, or pain with penetration on a scale from none (0) to severe (3) and identified 1 of these as their MBS for the trial outcome.
c - Vaginal Symptom Index = mean severity score of 5 vulvovaginal symptoms.
d - Among participants with a moderate or severe score at baseline.
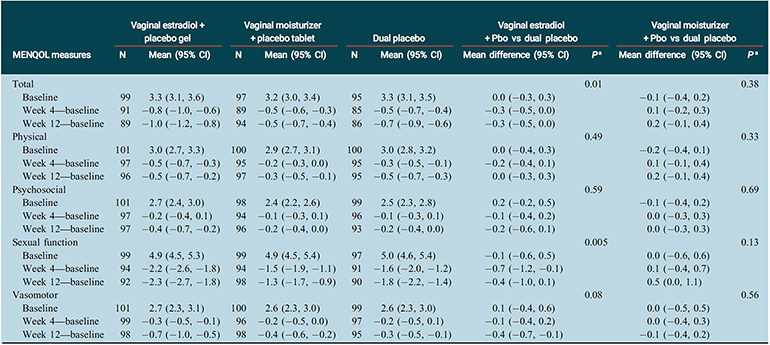
Fig. 2: Menopause-Specific Quality of Life Outcomes
Chart shows change in reported outcomes from baseline to week 4 and week 12.
Notes: CI, confidence interval; MENQOL, menopause-specific quality of life (range 1-8; 1¼best); Pbo, placebo.
aP values from contrasts comparing each treatment versus placebo in a repeated measures linear model of outcome as a function of randomization assignment, baseline value of the outcome measure, visit week (4 or 12) and clinical site.
To evaluate the efficacy of the interventions, the investigators administered standard questionnaires about sexual function, mood, quality of life and measured physical attributes such as vaginal maturation index and vaginal pH.
Encouraging Results
In the JAMA report of the impact of treatments on bothersome symptoms, results produced strongly positive and practical messages for women.
"Of every person in the study, the majority had an improvement in their symptoms—no matter what they were randomized to," Dr. Mitchell says. "This suggests significant relief is readily available, safe, and cheap for most women."
In terms of quality of life, the Menopause study showed that women who received the low-dose vaginal estradiol plus placebo improved slightly more than those receiving dual placebo. Dr. Mitchell says this suggests more nuanced effects of estrogen may need to be explored.
"Maybe, if we can better define the underlying biology of menopause," she says, "we might come up with a whole new treatment."
Many Women Do Not Seek Treatment
Both messages need to be communicated broadly among women and caregivers, says Dr. Mitchell, because most postmenopausal women do not seek treatment for their postmenopausal vulvovaginal symptoms.
"They just assume that it's part of aging for women and that there is nothing they can do about it," she says. "It is not talked about. This has to change."
Another reason women may not seek treatment for their symptoms is that they worry about ill-effects from the hormone estrogen. Dr. Mitchell believes these concerns are misplaced for most women taking the low dose used in the trial.
"Most people needn't be concerned as they are," Dr. Mitchell says. "Talk about it with caregivers, and also focus on the very positive messages of our work: that for most women, relief from symptoms is safe, cheap and easily available—and can be transformative."
Explore research in the Vincent Center for Reproductive Biology
Refer a patient to the Department of Obstetrics and Gynecology