Stratification Based on Predicted Survival Could Improve Randomization of ALS Trials
Key findings
- Stratification in clinical trials helps balance key baseline characteristics of the treatment groups, but only a few variables can be used, which limits the benefit in multifactorial diseases such as ALS
- In this study, stratification based on predicted survival improved the balance of key patient characteristics in treatment arms of simulated trials compared with traditional stratification
- The new stratification method was validated using an external dataset
- The use of predicted survival stratification substantially reduced the sample size needed to see a treatment effect in simulated trials
Because amyotrophic lateral sclerosis (ALS) is relatively rare, most clinical trials are small, which means randomization is more likely to fail. To avoid significant differences in key patient characteristics across treatment arms, investigators often stratify ALS patients on potential confounders, generally riluzole use or bulbar onset (versus limb onset).
Subscribe to the latest updates from Neuroscience Advances in Motion
James D. Berry, MD, MPH, co-director of the Massachusetts General Hospital Multidisciplinary Amyotrophic Lateral Sclerosis (ALS) Clinic, and colleagues have determined that stratifying patients by predicted survival should improve the randomization of ALS trials—and allows for reduced sample sizes needed. They describe their survival prediction model and methodology in Annals of Clinical and Translational Neurology.
The researchers searched the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) database in January 2016 and selected 4,882 patients whose records included baseline data on forced vital capacity (FVC) and either the ALS Functional Rating Scale (ALSFRS) or ALSFRS–Revised.
The team created a machine learning algorithm whose output was the log-likelihood of survival. Variables included in the algorithm were:
- Baseline total ALSFRS-R and calculated ALSFRS-R slope
- ALSFRS-R subscores
- Time since disease onset
- Time since diagnosis
- Baseline FVC
- Baseline weight
- Age
- Bulbar versus limb onset
- Study arm
- Gender
- Riluzole use
From the patient records, the researchers created "trial populations" of various sizes, from 48 to 224 patients. They developed 100 randomization schedules and performed 1000 to 10,000 randomizations on each population, using two stratification schemes:
- Predicted survival stratification using the algorithm
- Stratification on riluzole use and bulbar onset (traditional stratification)
On average, predicted survival stratification yielded 24% fewer randomization imbalances compared with traditional stratification. Randomization imbalances are statistically significant differences between two trial arms on any given patient characteristic.
Predicted survival stratification performed best when divided into tertiles: ≤33rd, >33rd to <66th, and ≥66th log-likelihood of survival percentiles. Tertile-based stratification was selected for in-depth analysis of a full panel of baseline patient characteristics and a wider range of trial population sizes.
In this analysis, predicted survival stratification was associated with an average 22% reduction in randomization imbalances (range, −16 to 49%) compared with traditional stratification.
The researchers validated their technique using an external dataset. When they applied the survival prediction algorithm to the placebo arm of the BENEFIT-ALS trial (n=279), randomization imbalances were reduced by an average of 26%.
Perhaps most important, the researchers say, is their finding that predicted survival stratification can improve the statistical power of a trial, thereby reducing the sample size needed. Using PRO-ACT data, they simulated trials of various sample sizes. They repeated each randomization 100 times, comparing predicted survival stratification with traditional stratification and no stratification.
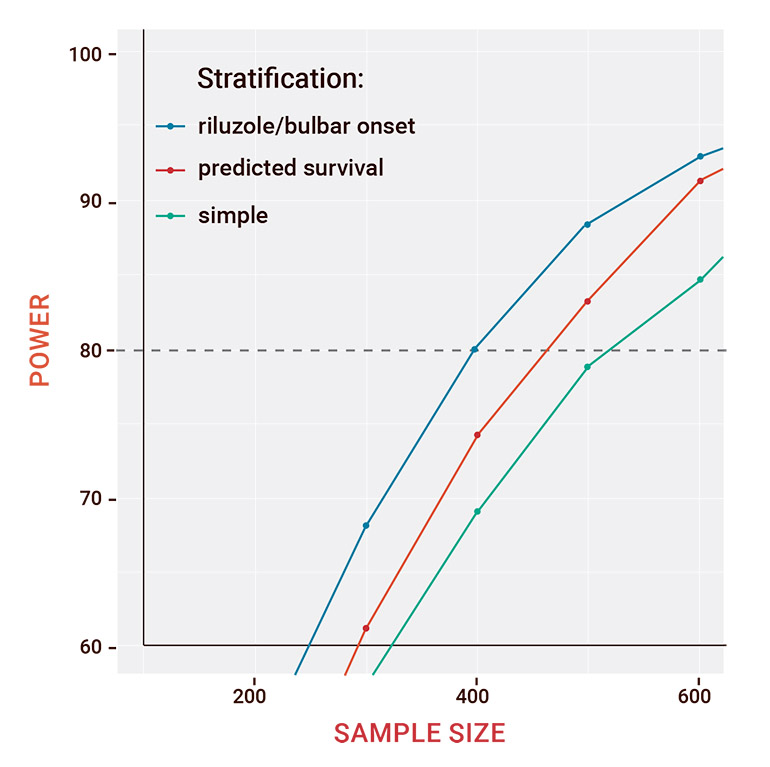
Figure 1: Plot of power versus sample size for a simulated treatment effect of extension of survival by 2.5 months
The researchers defined power as the proportion of 100 trials in which a regression test controlling for strata was able to detect the 2.5-month extension of survival in the simulated treatment group. The unstratified randomization crossed the 80% power threshold with a sample size above 500 patients. With traditional stratification, about 470 patients were needed. With predicted survival stratification, about 400 patients were needed (Figure 1).
Predicted survival stratification is ready to be adopted in prospective ALS trials, the researchers conclude. They say their methods could be adapted for use in other neurodegenerative diseases, including Alzheimer's, Parkinson's and Huntington's.
view original journal article Subscription may be required
Learn about the ALS Multidisciplinary Clinic at Mass General
Refer a patient for ALS care