Epigenetic Changes Drive Growth of SHD-Deficient GIST, Suggest Novel Treatment Approaches
Key findings
- About 15% of gastrointestinal stromal tumors (GISTs) are deficient in succinate dehydrogenase (SDH) and are notoriously drug-resistant
- This study showed that the characteristic hypermethylation in these tumors is associated with recurrent insulator losses, topological reorganization of the FGF3/FGF4 and KIT loci and potent induction of these oncogenes
- A mouse model of SDH-deficient GIST was highly sensitive to FGF receptor inhibition and even more sensitive to combined FGF receptor and KIT inhibition, suggesting potential novel treatment approaches
Subscribe to the latest updates from Oncology Advances in Motion
Gastrointestinal stromal tumors (GISTs) are typically caused by mutations of the KIT or PDGFRA oncogenes, which can be successfully targeted with existing drugs. However, about 15% of GISTs lack these mutations and have instead lost expression of succinate dehydrogenase (SDH), a key enzyme for cellular metabolism. This subset of GISTs is notoriously drug-resistant.
By investigating how SDH-deficient GISTs develop, Bradley E. Bernstein, MD, PhD, of the Center for Cancer Research at Massachusetts General Hospital, and colleagues have identified potential new treatment strategies. Their report appears in Nature.
Background
A clue to how SDH deficiency contributes to GIST development came from epigenetics, the study of external modifications to DNA that turn genes "on" or "off." An epigenetic process called DNA methylation is abnormally active in SDH-deficient tumors. Hypermethylation can disrupt "insulator" proteins that protect genes from inappropriate signals in their environment.
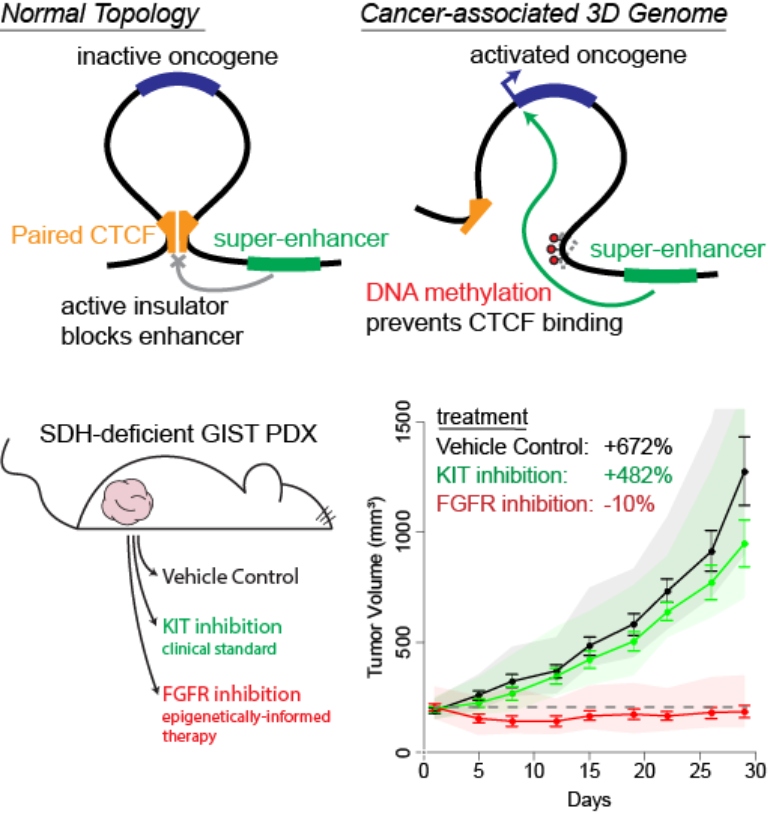
Figure 1
In healthy cells, cancer-causing oncogenes are protected by the formation of DNA "loops", which prevent them from being activated by nearby super-enhancer elements (top, right). In some cancer cells, these loops can be disrupted by epigenetic changes, exposing the oncogene to the activating super-enhancer. This results in tumor formation without direct mutation of the activated oncogene (bottom). Understanding the changes in these loops allows us to identify the activated cancer-causing oncogenes. Using a mouse model of one such tumor, we demonstrated that a drug blocking the effects of the activated oncogene could prevent tumor growth.
Disrupted Insulators
The researchers found that an insulator in the FGF3/FGF4 locus was lost in SDH-deficient GISTs. Recurrent loss of the insulator altered locus topology, allowing interaction between the oncogenes and a core GIST super-enhancer. Thus, the oncogenes were potently induced.
Likewise, recurrent insulator loss was apparent near KIT, with similar consequences.
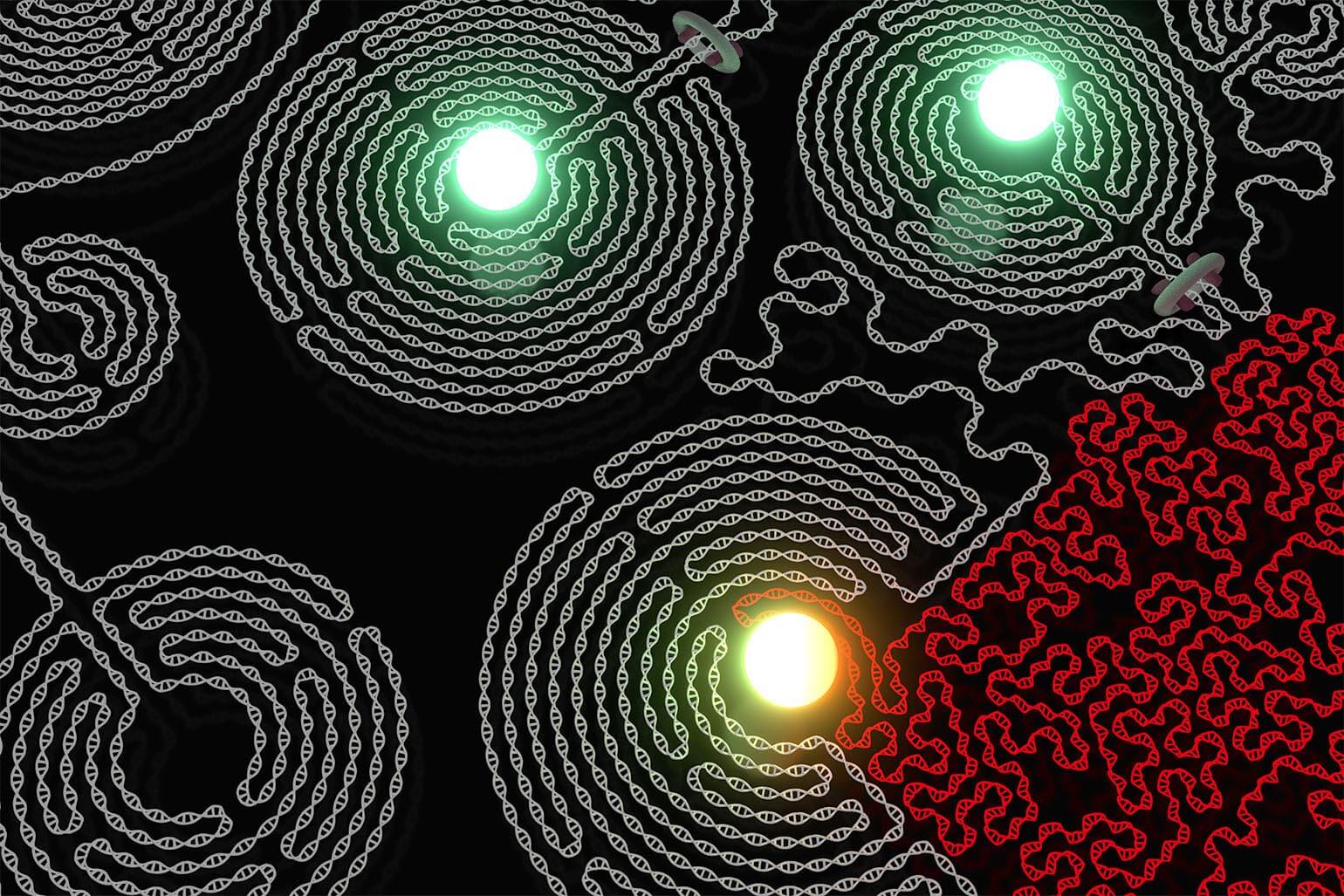
Figure 2
In cells, the DNA is formed into three-dimensional domains, also called "loops", by the insulator protein CTCF (bottom left, pink). These loops can protect and control the actions of activating elements known as enhancers (shown in center of the loop as the green/orange sphere).
Mouse Model
The investigators transplanted a patient-derived SDH-deficient GIST into mice and treated them with:
- Sunitinib, a KIT inhibitor approved for GIST
- BGJ-398, an investigational FGF receptor inhibitor
- The combination
Sunitinib minimally suppressed tumor growth, but BGJ-398 completely suppressed it throughout the dosing period. The combination suppressed tumor growth well beyond the dosing period.
Proof of Concept for Therapeutic Intervention
Tumor growth suppression with BGJ-398 suggests a critical role for FGF signaling in GIST tumorigenesis. The success of the combination therapy indicates that both the FGF and KIT pathways drive SDH-deficient GIST and supports the significance of the underlying epigenetic changes.
FGF receptor inhibitors, alone and in combination, deserve further testing in SDH-deficient GIST.
view original journal article Subscription may be required
Learn more about research at the Mass General Cancer Center
Refer a patient to the Mass General Cancer Center