Are The Two Newly Authorized COVID-19 Vaccines Safe and Effective?
The FLARE Four
- The FDA has now issued emergency use authorizations (EUA) for the mRNA-based SARS-CoV-2 vaccines made by Pfizer and Moderna
- Data from phase 3 trials, now publicly available, is consistent with remarkable efficacy across broad range of ages, comorbidities and risk of SARS-CoV-2 exposure
- Phase 3 data additionally show few significant safety concerns. But isolated reports of allergic reactions remind us of the possibility that widespread vaccination will reveal the occurrence of additional rare adverse events, as can occur with any vaccine
- Questions remain about the duration of immunity, efficacy/safety in certain groups (pregnant women, immunocompromised individuals, children and adolescents). Work is ongoing to obtain definitive answers to these important questions
Subscribe to the latest updates from Pulmonary & Critical Care Advances in Motion
On December 11, 2020, the Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for the Pfizer-BioNTech vaccine authorizing its use in individuals aged 16 years and older, and on December 18, 2020, the FDA issued an additional EUA for the Moderna vaccine for use in individuals aged 18 years and older. Both vaccines report efficacy of around 95% across different age groups, sex, ethnicity and comorbidity risk status, which is extremely encouraging as COVID-19 cases in the U.S. continue to climb.
Both the Pfizer/BioNTech and Moderna vaccines use a new messenger ribonucleic acid (mRNA) platform, whereby the mRNA encoding for the SARS-CoV-2 spike protein is enclosed in lipid nanoparticles for injection (as opposed to using another harmless virus as a carrier for the genetic instructions or injecting the recombinant spike protein directly). Once injected, mRNA is translated by host cells, and, ultimately, antigen-presenting cells that have acquired spike protein stimulate the adaptive immune system to produce spike protein-specific antibodies, priming it to counter future infections (Pardi et al. 2018).
There is also encouraging data (Sahin et al. 2020) that this approach generates not only an antibody response but also a robust Type 1 CD8 and CD4 T-cell response. This may be very important for long-term immunity and modulation of disease and likely makes escape mutants less likely.
Subsequently, the mRNA and spike protein are degraded by normal cellular processes. Animal data suggest that protein expression after intramuscular (IM) injection lasts for about 10 days and may also occur in some distant sites such as the liver for a few days (Pardi et al. 2015). mRNA is structurally distinct from DNA (deoxyribonucleic acid), does not enter the nucleus and cannot integrate into the genome.
While the Pfizer vaccine is the first mRNA vaccine to receive authorization for use outside a clinical trial, animal studies investigating the potential of mRNA vaccines date back to at least 2001, and there are multiple mRNA vaccine candidates for infectious diseases and cancers currently in phase 1 and 2 clinical trials (reviewed in Zhang et al. 2019; Pardi et al. 2018). The first report of successful transcription of mRNA administered in vitro was published in 1990 by Woff and colleagues (Wolff et al. 1990), although issues with mRNA stability initially limited the development of this technology.
Both vaccines utilize a "prime-boost" strategy (Woodland 2004) of two injections, spaced three to four weeks apart. This approach has been shown to enhance antibody response (Graham et al. 2020) to some COVID-19 vaccines and is thought to also enhance T-cell responses and lead to longer-lasting immunity.
A major difference between the two vaccines is the storage conditions: the Pfizer vaccine requires ultracold storage, while the Moderna vaccine is stable under standard freezer and refrigerator conditions. mRNA is normally easily degraded, but can be stabilized with modifications to the backbone structure or lipid formulation. Because specific nanoparticle formulations are proprietary, it is not known precisely why the storage conditions differ for the two vaccines. Moderna has somewhat more experience with the mRNA platform and may have optimized its formulation for better stability.
It's worth noting that there are other mRNA vaccines in clinical trials that also report stability at higher temperatures; for instance, a lyophilized rabies mRNA vaccine developed by CureVac (which recently began phase 2b/3 trials of its COVID-19 vaccine candidate) reported stability at 5-25C for 36 months, and at 40C for six months (Alberer et al. 2017).
FDA Review Process
As discussed in a previous FLARE on November 19, 2020, the typical vaccine development process has been expedited due to the public health emergency. Part of this accelerated timeline comes from increased and shared resources for research, as well as running development steps simultaneously rather than sequentially. In June, 2020, the FDA released guidance outlining the data and information they would need in order to consider issuing an EUA; this includes preclinical and clinical data, as well as details about the manufacturing process and facilities to ensure that the vaccines can be produced reliably and consistently. It is worth noting that, according to Stephen Hahn, the FDA commissioner, the FDA is one of the few medical regulatory agencies that independently re-analyzes the raw data for the application, rather than relying on the analyses presented by sponsors.
The FDA consults with the Vaccines and Related Biological Products Advisory Committee (VRBPAC), which is a panel of outside independent technical experts who provide feedback on the scientific data and public health significance of a proposed vaccine. VRBPAC meetings are open to the public and streamed online, with meeting materials made available at least two business days prior to the meeting. They met on December 10, 2020 to discuss Pfizer's EUA application, and on December 17, 2020 to discuss Moderna's application. The FDA considers, but is not bound by, the input received from the VRBPAC.
While the EUA allows manufacturers to begin distributing and vaccinating the general public, it is expected that companies will continue to collect placebo-controlled data in ongoing trials for as long as feasible and eventually submit a Biologics License Application (BLA) for full licensing and approval to market and distribute. A BLA requires a comprehensive submission including preclinical and clinical data, with information and details of the manufacturing process and facilities as well as a preapproval inspection of manufacturing sites. The FDA issues EUAs for medical products to be used in an emergency situation when there are no adequate, approved alternatives. In general, the legal threshold for granting an EUA is lower compared to a BLA, however, the FDA has stated that the standards for an EUA for a COVID-19 vaccine will be similar to what normally would be needed for a BLA, albeit with a shorter duration of safety data, since it would be given to potentially millions of uninfected, otherwise healthy individuals.
How Does the Data Look?
Pfizer has now published (Polack et al. 2020) its phase 2 and 3 trial data in the New England Journal of Medicine, and the FDA has released its independent analysis of the Pfizer data. Moderna has not yet published a manuscript of the data from their phase 3 trial but an independent FDA analysis of the data has been made public.
Study Demographics
Age, race and underlying or preexisting medical conditions have been found to be risk factors for developing severe COVID-19, and thus there is particular interest in ensuring that a vaccine will protect these vulnerable populations. In the Pfizer trial, 21% of participants were over the age of 65, 28% identified as Hispanic or Latinx, 9.3% identified as Black or African American, 70% were obese or overweight (as measured by body mass index), and 21% had at least one co-existing condition such as diabetes or chronic pulmonary disease. The Moderna trial had similar demographics: 25% of participants were over the age of 65, 20% identified as Hispanic or Latinx, 9.7% identified as Black or African American, and 22% had one or more high-risk conditions. Additionally, in the Moderna trial, 25% of participants were health care workers, and overall 82% of participants were considered at occupational risk for SARS-CoV-2 exposure.
How Safe Are the Vaccines?
The mRNA vaccine platforms are new technology. This has led to concerns, particularly in the popular press, that the rapid development process will lead to less stringent evaluation of safety. In this context, it is worth noting that the process of mRNA transcription and protein expression mimics the process that occurs in natural infection with any virus and, on this basis, it is reasonable to expect the immune response will be essentially similar as well (Zhang et al. 2019). Moreover, emerging clinical trial data is consistent with this expectation.
Both the Pfizer and Moderna vaccines seem to be well tolerated serious adverse events (SAEs) were uncommon (<1%) in both trials, without meaningful imbalances between the vaccine and placebo arms. For both vaccines, younger participants were more likely to report adverse reactions compared to older participants (defined as over 55 years old for Pfizer, and over 65 years old for Moderna), and adverse reactions were more common after the second injection compared to the first. Rates of adverse reactions are generally similar between Pfizer and Moderna. The most common local reaction was mild to moderate injection site pain, and common systemic events included fatigue, headache, muscle aches, joint pain and chills. Overall, side effects were generally considered mild to moderate in severity and resolved within a few days, but it will be important to be clear about the expected side effects with providers and patients so that people are not surprised and are not discouraged from returning for the second injection.
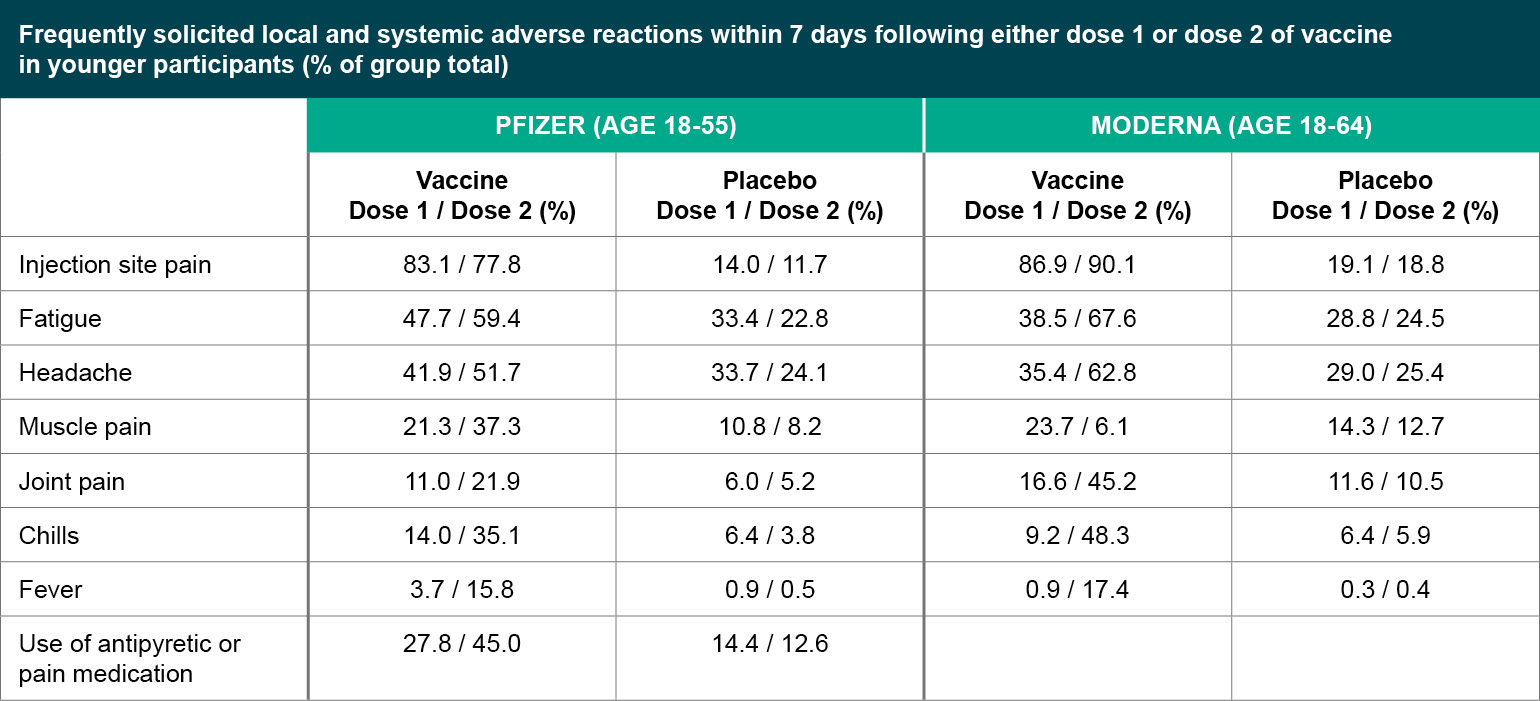
Figure 1
Frequently solicited local and systemic adverse reactions within 7 days following either dose 1 or dose 2 of vaccine in younger participants (% of group total).
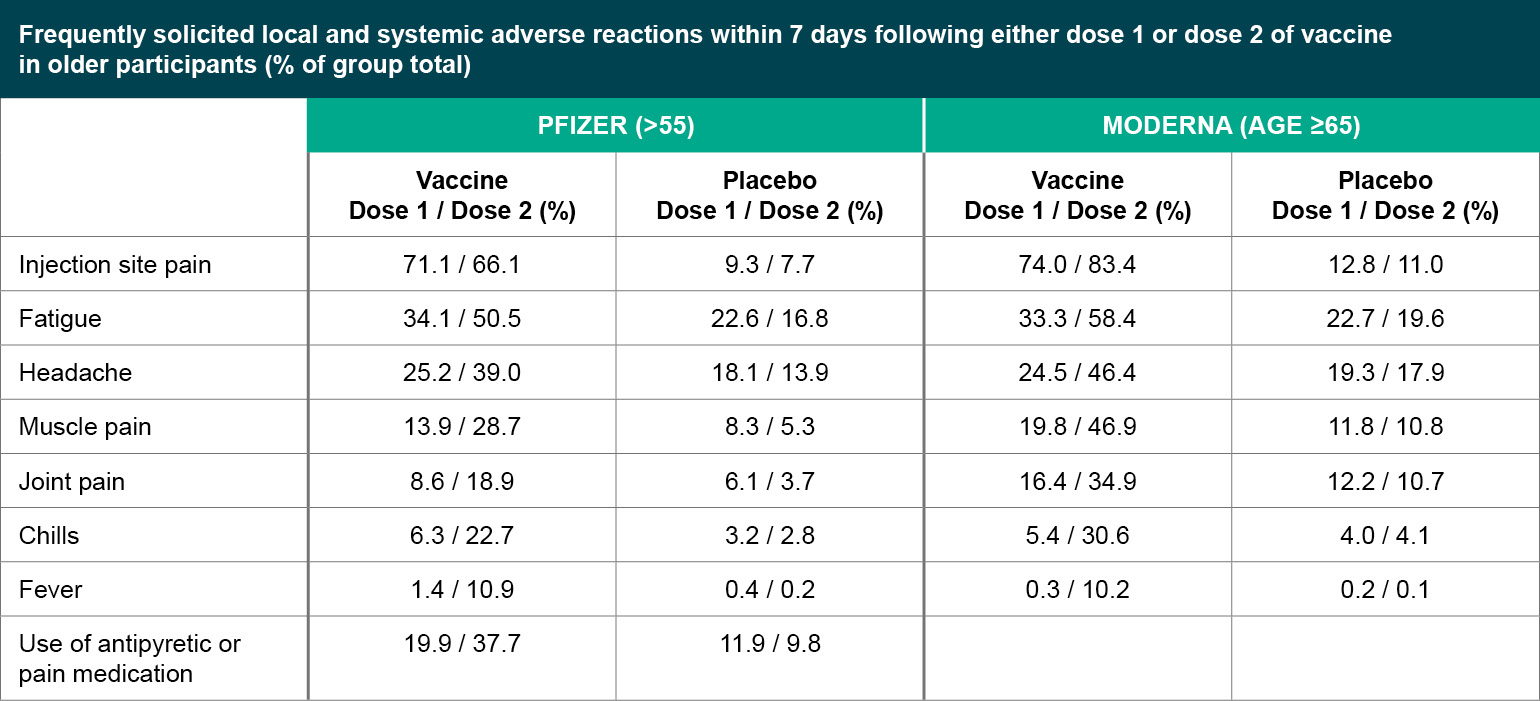
Figure 2
Frequently solicited local and systemic adverse reactions within 7 days following either dose 1 or dose 2 of vaccine in older participants (% of group total).
Given the balance between treatment and placebo arms in terms of adverse events, it is fair to ask if all of the adverse events were related to vaccination. Among serious, non-fatal serious adverse events (SAEs), the FDA ruled that two of the SAEs in the Pfizer trial (shoulder injury and lymphadenopathy) may be related to the vaccine. For Moderna, the FDA ruled that three of the SAEs were likely related to the vaccine, including intractable nausea/vomiting and two reports of facial swelling in participants with a previous history of cosmetic filler injections. There were also SAEs for which the contribution of the vaccine could not be excluded, including rheumatoid arthritis, peripheral edema/dyspnea with exertion and autonomic dysfunction. Four vaccine recipients in the Pfizer study, and three vaccine recipients and one placebo recipient in the Moderna study reported Bell's palsy (facial paralysis). While the observed frequency is consistent with the expected background rate in the general population, in both cases, the FDA recommended continued surveillance for cases of Bell's palsy with vaccine deployment.
Allergic Reactions
Since the Pfizer vaccine has been deployed in the U.K. and U.S., two health care workers in the U.K. developed a severe allergic reaction after receiving the vaccine, and as of December 19, 2020, the CDC has identified six case reports of a severe allergic reaction. The two British health care workers reportedly had a history of severe allergies to food or medications, but only one American case is reported to have a history of anaphylaxis following rabies vaccination. Notably, these reactions occurred on first exposure to the vaccine, which raises the possibility of allergy to non-RNA components of the vaccine and non-IGE mediated reactions. British regulators now recommend against giving the vaccine to anyone who has ever had an anaphylactic reaction to a food, medicine or vaccine. The FDA in contrast has stated that people with severe food or environmental allergies should be able to get the shot, but people who have had a severe allergic reaction to any ingredient of the vaccine, or a severe allergic reaction after a previous dose of this vaccine, should not receive the shot. In particular, polyethylene glycol, which is a component of both vaccines and a component in other injectable medications and cosmetics, has been suggested as a possible trigger, although the mechanism is unclear and some scientists remain skeptical. The CDC recommends observing patients for 15 minutes after vaccination, or 30 minutes for patients with a history of severe allergic reactions, to monitor for immediate adverse reactions.
It is important to recognize that, as with any vaccine, there is the possibility of very rare reactions that were not seen in clinical trials due to the limited number of participants. This is not specific to the EUA process but simply reflects the fact that very rare events require a very large number of vaccinations to manifest. As of Monday, December 21, 2020, 614,117 doses of the Pfizer vaccine had been administered in the U.S., and the CDC reports six cases of severe allergy. The clinical trials enrolled approximately 30,000 to 40,000 participants, with no reports of anaphylactic or severe hypersensitivity reactions; around 1% of participants in both trials reported hypersensitivity-related adverse events, including injection site rash, hives and rash maculo-papular. The risk posed to any individual by these rare events must, of course, be balanced against the (likely higher) risk of SARS-CoV-2 infection.
Pregnancy and Reproduction
In both the Pfizer and the Moderna study, participants were screened for pregnancy prior to each vaccination, and a positive test resulted in exclusion or discontinuation from the study. As of the respective data cut off dates (November 14, 2020, for Pfizer, December 2, 2020, for Moderna), 23 pregnancies were reported in the Pfizer study (12 vaccine, 11 placebo) and 13 pregnancies were reported in the Moderna study (6 vaccine, 7 placebo). Among those participants, unsolicited adverse events related to pregnancy include spontaneous and elective abortions, and retained products of conception, all in the placebo group. Pregnancy outcomes are otherwise unknown at this time for both studies.
Moderna submitted data from a reproductive toxicity study in rats that showed that the vaccine given prior to mating and during gestations periods did not have any adverse effects on female reproduction, fetal or embryonic development or postnatal development, except for skeletal variations which are common and typically resolve postnatally without intervention. Pfizer did not submit animal data, but studies are ongoing.
How Well Do They Work?
The Pfizer study defined their primary endpoint as cases of symptomatic, confirmed COVID-19 occurring at least seven days after the second injection, while Moderna counted symptomatic, confirmed COVID-19 cases occurring at least 14 days after the second injection. Both studies exceeded the number of cases they estimated would be needed to provide sufficient power for statistical analyses and in a shorter amount of time than originally anticipated, due at least in part to the uncontrolled spread of COVID-19 in the community. Both studies report approximately 95% vaccine efficacy.
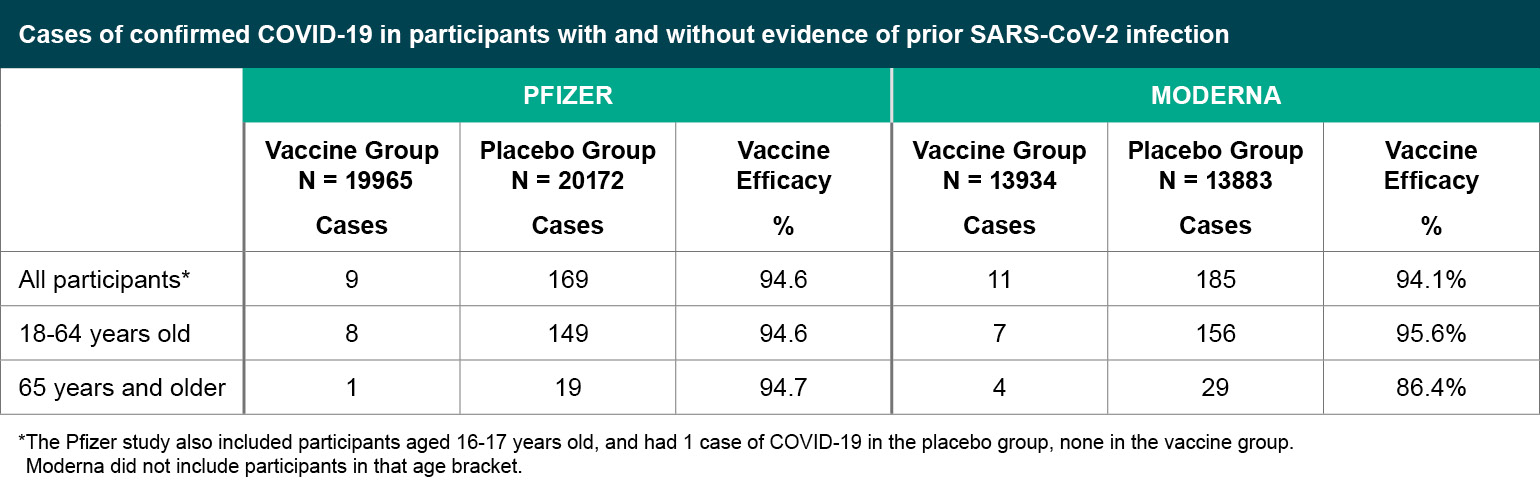
Figure 3
Cases of confirmed COVID-19 in participants with and without evidence of prior SARS-CoV-2 infection.
*The Pfizer study also included participants aged 16-17 years old, and had 1 case of COVID-19 in the placebo group, none in the vaccine group. Moderna did not include participants in that age bracket.
Both the Pfizer and Moderna vaccine efficacies were similar across subgroups including age, sex, ethnicity (i.e., identifying as Hispanic or Latino) and comorbidity status (e.g., having at least one of the Charlson comorbidity index category or obesity, placing them at higher risk of severe COVID-19). The total case numbers broken down by race were low, with the exception of subjects identifying as white, but suggest similar efficacy across racial groups (e.g., Pfizer had seven cases in subjects identifying as Black or African American, and Moderna had four; in both studies, the cases in Black or African American subjects were all in the placebo group).
In terms of preventing severe COVID-19, which was a secondary efficacy endpoint in both studies, subjects who met the severe disease classification seven or more days after the second dose were predominantly in the placebo group. For Moderna, 30 of the cases were classified as severe based on low oxygen saturation <93% on room air without any other severe disease criteria, all in the placebo group. For Pfizer, one vaccine recipient and four placebo recipients met the severe case definition based on low oxygen saturation < 93% on room air, without other severe disease criteria.
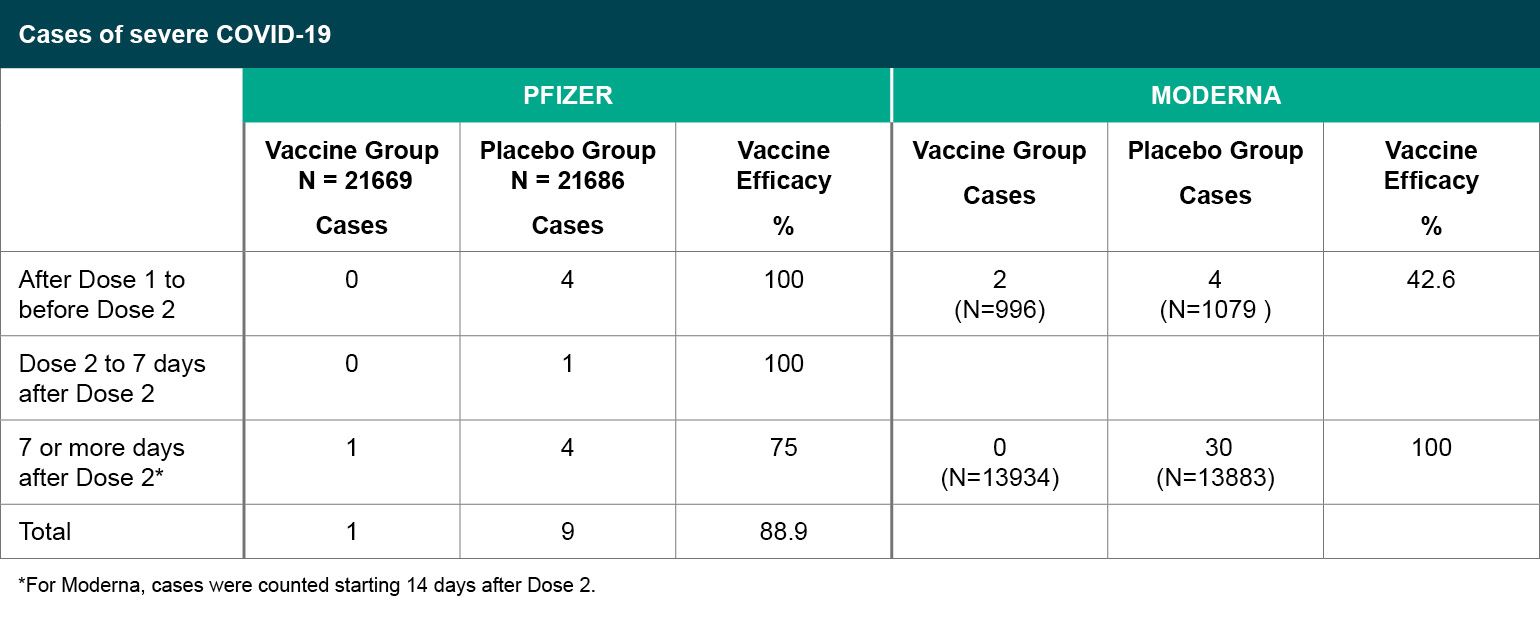
Figure 4
Cases of severe COVID-19.
*For Moderna, cases were counted starting 14 days after Dose 2.
While neither study was designed to look at vaccine efficacy after a single injection, an examination of COVID-19 cases between the two doses showed vaccine efficacy of 52% for Pfizer (39 cases in the vaccine group and 82 in the placebo group) and 80.2% for Moderna (seven cases in the vaccine group, 39 in the placebo group). COVID-19 case numbers between vaccine and placebo groups began to diverge around day 12 for Pfizer, and day 14 for Moderna, suggesting an early onset of a partially protective effect of immunization. Given that adaptive immunity takes at least seven days to develop, the above analysis, which looked at protection after one dose and included the first week of data, likely underestimates the level of protection afforded by one dose.
What If You Had COVID-19 Already?
In terms of past COVID-19 infection, neither study was designed to look at efficacy in people with prior SARS-CoV-2 infection; in both studies, only around 5% of participants had either positive or unknown evidence of prior SARS-CoV-2 infection. Only six of the confirmed COVID-19 cases in the Pfizer study were in subjects with either unknown or confirmed prior history of COVID-19 (one in the vaccine group, five in the placebo group). Moderna had only two cases, both in the placebo group, where the subject had either an unknown or confirmed prior history of COVID-19. The limited data suggests there can be reinfection and that the vaccine can be protective. Interestingly, the Moderna data suggest that participants with a positive baseline evidence of prior SARS-CoV-2 infection were less likely to report adverse reactions to the vaccine; Pfizer did not provide that subanalysis.
What Are the Knowledge Gaps?
While the current data for both vaccines are very promising, there are still many unknowns. The Pfizer trial included 196 participants who were HIV-positive but those data from those individuals were not included in the NEJM paper, and overall, the group was too small to evaluate efficacy outcomes. So far there is limited to no data on the effects on pregnant or lactating individuals, nor safety and efficacy for immunocompromised people or adolescents, as these groups were excluded from the phase 3 trials. Moderna has initiated a combined phase 2 and 3 clinical trial to test its vaccine in adolescents aged 12 to 18, and Pfizer has also enrolled adolescents aged 12 to 15. It is important to remember that the 95% efficacy data is based on a median of two months of observation, under current conditions in a society where exposure is limited. It is unknown at this time how long the protection will last, and it is unclear whether the vaccine prevents asymptomatic infection and viral transmission. It will be important to continue the placebo-controlled trial for as long as possible so that this data can be collected, however, there are of course ethical concerns given the risk of SARS-CoV-2 infection and the demonstrated efficacy of the vaccine.
Plans for Placebo-controlled Follow-up
Both Pfizer and Moderna expect that participants may request unblinding in order to receive an EUA vaccine. In particular, 25% of participants in the Moderna study are health care workers. Pfizer plans to provide the vaccine to placebo recipients upon request according to local or national recommendations, as well as automatically offering the vaccine to placebo recipients after six months of follow up if participants have not already requested unblinding. Both plan to continue following placebo participants who subsequently receive the vaccine for a full 18 to 24 months to continue collecting safety and efficacy data.
Conclusions
The Pfizer and Moderna vaccines are the first mRNA-based vaccines to be authorized for use outside a clinical trial. The fact that two vaccines using this new vaccine platform, developed independently, show similar safety and efficacy data is encouraging, although long-term follow-up will be important. AstraZeneca and Janssen/Johnson and Johnson trials are still ongoing. Currently, the U.K.'s Medicines and Healthcare products Regulatory Agency (MHRA; their equivalent of the FDA) is reviewing the data for AstraZeneca and is anticipated to reach a decision by early January 2021. AstraZeneca has enrolled 26,000 participants for its U.S. trial, nearing its goal of 30,000 people. It is likely they will be ready to apply for EUA in the U.S. in late January 2021. Janssen/Johnson and Johnson has completed enrollment of its phase 3 trial and also anticipates applying for EUA by the end of January 2021. Dr. Moncef Slaoui, co-leader of Operation Warp Speed, has said that approval of AstraZeneca and Janssen's vaccine will be important for achieving the goal of vaccinating all Americans by summer 2021.
References
Alberer, Martin, Ulrike Gnad-Vogt, Henoch Sangjoon Hong, Keyvan Tadjalli Mehr, Linus Backert, Greg Finak, Raphael Gottardo, et al. 2017. "Safety and Immunogenicity of a mRNA Rabies Vaccine in Healthy Adults: An Open-Label, Non-Randomised, Prospective, First-in-Human Phase 1 Clinical Trial." The Lancet. 390 (10101): 1511–20.
Graham, Simon P., Rebecca K. McLean, Alexandra J. Spencer, Sandra Belij-Rammerstorfer, Daniel Wright, Marta Ulaszewska, Jane C. Edwards, et al. 2020. "Evaluation of the Immunogenicity of Prime-Boost Vaccination with the Replication-Deficient Viral Vectored COVID-19 Vaccine Candidate ChAdOx1 nCoV-19." NPJ Vaccines. 5 (July): 69.
Pardi, Norbert, Michael J. Hogan, Frederick W. Porter, and Drew Weissman. 2018. "mRNA Vaccines - a New Era in Vaccinology." Nature Reviews. Drug Discovery 17 (4): 261–79.
Polack, Fernando P., Stephen J. Thomas, Nicholas Kitchin, Judith Absalon, Alejandra Gurtman, Stephen Lockhart, John L. Perez, et al. 2020. "Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine." The New England Journal of Medicine. December. https://doi.org/10.1056/NEJMoa2034577.
Wolff, J. A., R. W. Malone, P. Williams, W. Chong, G. Acsadi, A. Jani, and P. L. Felgner. 1990. "Direct Gene Transfer into Mouse Muscle in Vivo." Science. 247 (4949 Pt 1): 1465–68.
Woodland, David L. 2004. "Jump-Starting the Immune System: Prime-Boosting Comes of Age." Trends in Immunology. 25 (2): 98–104.
Zhang, Cuiling, Giulietta Maruggi, Hu Shan, and Junwei Li. 2019. "Advances in mRNA Vaccines for Infectious Diseases." Frontiers in Immunology. 10 (March): 594.
View all FLARE content
Learn more about research in the Division of Pulmonary and Critical Care Medicine